Chemistry Chat
- Focusing on the Elements - Compounds Composed of Two Elements (4)
We have covered compounds consisting of carbon and one other element so far. Let us continue covering bielemental compounds and take a look at the family of nitrogen oxides this time. Nitrogen oxides are collectively called NOx and infamously known as the cause of air pollution. Nevertheless, their structures are diverse and there are many important examples.
Dinitrogen monoxide (N2O)
Dinitrogen monoxide (N2O), which is also known as nitrous oxide, is a colorless gas. It has resonance structures as shown below.
Nitrous oxide was discovered in 1772 by Joseph Priestley in England. Because the inhalation of the gas causes drunk feeling and cramping of facial muscles (which causes smiley expression), it is called “laughing gas.” Back then, N2O was a popular recreational gas and there were said to be shows featuring the euphoric effects of inhaling it. The participants of the show laughed out loud on the stage, walked around randomly while bowing repeatedly, stepped into the audience and tried to walk over them, so these shows must have been quite chaotic.
However, people began to learn to use the biochemical effects of N2O for more productive purposes, such as for anesthesia during dental treatment. For thousands of years, the patients of surgical operations had had to endure the pain, so the advent of anesthesia by laughing gas was a historic discovery. Many other analgesic substances were introduced later, but nitrous oxide is still used today for its potent pain-relieving effect.
On the other hand, nitrous oxide is known as the strongest ozone-destroying substance and also has about 300 times stronger greenhouse effect than carbon dioxide. Considering the impact it has on the environment, it is one of the gases we should avoid as much as possible.
Page Top
Nitrogen Monoxide (NO)
Nitrogen monoxide (NO) is also a colorless gas, which is produced by reacting nitrogen and oxygen under high temperature conditions. Another way of generating it is from the reaction between copper and dilute nitric acid, which is an experiment some people might remember from middle school chemistry class.
The first person who synthesized nitrogen monoxide is considered to be the sixteenth-seventeenth century Belgian scientist Jan Baptist van Helmont, who is also known to have coined the word gas from chaos. However, the aforementioned Joseph Priestley is generally credited as the one who analyzed it carefully and left a systematic record.
Nitrogen monoxide is toxic and inhaling it causes symptoms of the central nervous system and the loss of consciousness in just a few minutes. It is oxidized in the body to produce nitric acid and nitrous acid, both of which can damage the respiratory organs. It is a rather scary substance, though it may not be as serious as nitrogen dioxide.
Nitrogen monoxide, however, has important biological functions too. For example, it helps increase the blood flow by relaxing the smooth muscle of blood vessels. The reason that nitroglycerin and amyl nitrite are used as the medications of coronary heart disease is because these prodrugs decompose to produce nitrogen monoxide in the body. Interestingly, this mechanism of action is associated with how other drugs work too, including Viagra.
The role of nitrogen monoxide, which is naturally a toxic gas, as a biological signaling agent in the cardiovascular system was discovered by Louis Ignarro, Rober Furchgott, and Ferid Murad. The three scientists were awarded the Nobel Prize in Physiology or Medicine in 1998, which was undisputable considering the significance of the discovery.
Nitrogen monoxide is also involved in the activation of the immune system. Macrophages, which are one of the immune cells, produce a large amount of nitrogen monoxide to kill pathogens. However, for septic patients, generation of too much NO can lead to excessive vasodilation and dangerous levels of hypotension. Despite being consisting of only two atoms, the biological role of nitrogen monoxide is surprisingly profound.
Nitrogen Dioxide (NO2)
Just like nitrogen monoxide, nitrogen dioxide is a stable free radical species. It is a rare gas noticeably colored brown, and the color originates from its structure in which the unpaired electron is delocalized throughout the molecule. The unpaired electron is also the reason why the molecule is V-shaped at the central nitrogen atom, unlike the linear molecule of carbon dioxide.
Besides having a high inflammatory effect on the respiratory system, NO2 has a property to hinder the transport of oxygen by competitively binding to hemoglobin. It also reacts with oxygen in the air promoted by ultraviolet irradiation to produce toxic ozone, which is the main cause of so-called photochemical smog. When it is dissolved in water, NO2 becomes nitric acid and nitrous acid, thus is responsible for acid rain. In addition, it is a strong oxidant that can form explosive substances upon contact with flammable compounds such as hydrocarbons. All in all, this is one of the least environmentally friendly substances.
In a laboratory, it can be generated by adding concentrated nitric acid to copper. In nature, it is formed by processes such as lightning-induced reactions and carried to the ground dissolved in rainwater. The resulting nitrate salts are absorbed by plants as nutrients and become a part of biological constituents such as proteins. This is an important nitrogen fixation process occurring in the natural world.
Page Top
Dinitrogen Tetroxide (N2O4)
There is an equilibrium between nitrogen dioxide and dinitrogen tetroxide, which shifts towards the latter at low temperatures. Dinitrogen tetroxide is no longer a free radical species and has no color, but it still tends to be slightly colored due to the small amount of nitrogen dioxide present in the mixture.
One of the important applications of N2O4 is its use as a rocket propellant. As a strong oxidizer, N2O4 reacts with hydrazine compounds, the fuel, to form a “hypergolic” propellant. It was used for sending rockets such as the American Titan and the Chinese Chang Zheng out of the stratosphere, making contributions to the outer space development. However, it is highly toxic and there has been a concern about its impact on the environment.
Dinitrogen Trioxide (N2O3)
Although not as well-known as the ones we have seen so far, dinitrogen trioxide (N2O3) also exists. It is formed when nitrogen monoxide and nitrogen dioxide are combined at low temperatures. In gas phase, it dissociates back to NO and NO2, so it can only exist as either liquid or solid.
As shown below, the structure of N2O3 is the one that contains an N–N bond, and the other plausible structure corresponding to nitrous acid anhydride (O=N–O–N=O) is not known. The latter is probably too unstable to exist long enough to be detected.

Dinitrogen Trioxide
Dinitrogen Pentoxide (N2O5)
Unlike the other NOx compounds, dinitrogen pentoxide is a solid at room temperature because it is actually an ionic species consisting of NO3- and NO2+ ions. In gas phase, it exists as a neutral molecule with the structural formula of O2N–O–NO2. Its handling requires care, however, since it spontaneously decomposes to nitrogen dioxide and oxygen even at room temperature.
This molecule corresponds to the anhydride of nitric acid, and it can be obtained by dehydration of nitric acid by phosphorus pentoxide. It gradually reverts back to nitric acid when it reacts with the moisture in the air.
Page Top
Trinitramide (Tetranitrogen Hexoxide, N4O6)
The latest addition to the nitrogen oxide family is trinitramide having the molecular formula of N4O6. It was the first member in 170 years, with the last one preceding it being dinitrogen pentoxide discovered in 1840. As you can see in the picture below, it can be called the triamide form of nitric acid. It was synthesized by the reaction of potassium dinitramide (KN(NO2)2) with nitronium tetrafluoroborate (NO2BF4) at a low temperature. In contrast to dinitrogen tetroxide, which has a completely planar structure, trinitramide has a propeller-like twisted shape.
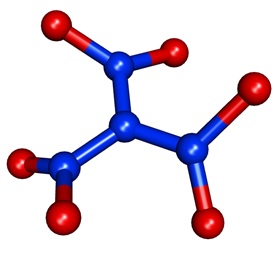
Trinitramide
It is obviously an extremely high-energy compound and it is too unstable to exist at room temperature. As a rocket propellant oxidizer, it is thought to be 20 to 30% more efficient than its conventional counterparts, and its chlorine-free nature makes it environmentally friendlier. It is possible that rockets in near future will fly to the outer space using this compound as a fuel component.
The structural possibility of nitrogen oxide compounds is wide open on paper, but actual synthesis of them is probably among the most challenging. Let us wait for the next member to join in, though it may take a long time.
Page Top
Introduction of the author
Kentaro Sato
[Brief career history] He was born in Ibaraki, Japan, in 1970. 1995 M. Sc. Graduate School of Science and Engineering, Tokyo Institute of Technology. 1995-2007 Researcher in a pharmaceutical company. 2008- Present Freelance science writer. 2009-2012 Project assistant professor of the graduate school of Science, the University of Tokyo. 2014-present Publicist for π-system figuration, scientific research on innovative areas.
[Specialty] Organic chemistry
Page Top
![]()



