Chemistry Chat
- Focusing on the Elements - Compounds Composed of Two Elements (1) Halocarbons
Carbon can form stable structures of any length, can bond with many other elements, and can form single, double, and triple bonds. Needless to say, these unique characteristics of carbon are the foundation of the chemical diversity in this world. In fact, one can think of virtually infinite number of compounds from just the combination of two elements, carbon and hydrogen.
Take simple alkanes as examples. The number of isomers is calculated to be 75 for decane (C10), 366,319 for icosane (C20), and 4,111,846,763 for triacontane (C30) (Note, however, that these numbers do not include enantiomers and other stereoisomers. They are also theoretical numbers, meaning that they count isomers that cannot actually exist for reasons such as excessive steric hindrance.) When additional factors like multiple bonds and cyclic structures are taken into account, it is not difficult to imagine how astronomically huge the number of hydrocarbons can be.
Fluorocarbons
In terms of chemical diversity, the combination of which two elements do you think comes in second behind hydrocarbons? The first one to come to your mind may be the carbon-halogen combination. For instance, fluorine has a similar atomic radius to hydrogen and C-F bond is also extremely strong, so fluorocarbons have a possibility to form as many compounds as hydrocarbons.
Fluorocarbons were synthesized in 1926 for the first time. The fact that it took 40 years after the isolation of fluorine is probably a testament to how difficult the handling of elemental fluorine was. The first fluorocarbon synthesized was the simplest of them all, carbon tetrafluoride (CF4).
In 1938, Teflon was serendipitously discovered in the laboratory of DuPont, when a sample of tetrafluoroethylene (F2C=CF2) stored in a metal cylinder was found to have polymerized. Teflon is resistant against thermal and chemical stresses as well as having very low friction. These properties have made it an essential coating material for household commodities such as kitchenware as well as laboratory equipment.
Perfluorinated alkanes started to draw the interest of organic chemists with the introduction of the field called fluorous chemistry. Highly fluorinated (“fluorous”) compounds tend to be insoluble in common organic solvents and water but soluble in perfluoroalkane solvents. This is a useful property in terms of the isolation of relevant compounds. As fluorous solvents, perfluorohexane (F3C(CF2)4CF3) and perfluorotoluene (C6F5CF3) are used frequently.
Some of these fluorocarbons, however, possess strong biological toxicity. For example, perfluoroisobutene is about ten times more toxic than phosgene and is even included in the list of substances regulated by the Chemical Weapons Convention. The hight electronegativity of fluorine makes perfluoroisobutene a potent electrophile that can damage organs such as the lungs. One must always be aware of the potential toxicity of polyfluoroolefins when there is a need to use them.

Perfluoroisobutene
Fluorinated fullerenes are worth mentioning as unique examples among fluorocarbons. For instance, the Russian research team led by Olga V. Boltalina has reported a number of halogenated fullerenes which they synthesized by treating fullerene with various halogenation reagents. As shown below, in a molecule of C60F18 the attachment of 18 fluorine atoms has resulted in this humorous shape reminiscent of jellyfish.
C60F18 has a humorous shape reminiscent of jellyfish
Page Top
Chlorocarbons
How about the combination of C-Cl, with chlorine being a size larger than fluorine in the periodic table? It may appear that one could make as many compounds as fluorocarbons, but it turns out that it is not the case in reality. As for perchloroalkanes, perchlorobutane containing only 4 carbons is the largest that has ever reported. The fact that C-Cl bond is much weaker compared with C-H and C-F bonds coupled with other reasons such as the large atomic radius of chlorine causing steric hindrance seem to prevent larger perchloroalkanes from existing as stable compounds.
Among chlorocarbon compounds, carbon tetrachloride and tetrachloroethylene are familiar to chemists as solvents. But of course, these chlorinated solvents are getting more and more strictly regulated due to their toxicity and negative environmental impact.
Unlike perchloroalkanes, many perchlorinated derivatives of aromatic compounds are known thanks to reduced steric hindrance among the chlorine atoms. Hexachlorobenzene (C6Cl6) was once used as an agricultural pesticide and an antiflamming agent for clothes, but it is banned today because of its carcinogenicity.
In addition to hexachlorobenzene, perchlorinated versions of pyrene, triphenylene, fluoranthene, and coronene have been synthesized.

Perchlorinated versions of pyrene, triphenylene, fluoranthene, and coronene
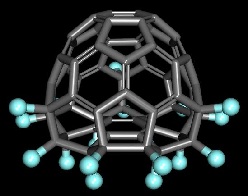
Like the aforementioned fluorinated fullerene, there are interesting chlorinated fullerene derivatives. For example, the treatment of fullerene with antimony pentachloride yielded several chlorinated fullerenes, of which C60Cl30 has a drum-like shape as shown in the figure below.

C60Cl30 has a drum-like shape
C50Cl10 is another example which was synthesized in 2004. This one looks kind of like Saturn as shown below. C50 fullerenes are usually too strained to be isolated. However, this rare C50 derivative was directly obtained by the arc discharge synthesis in the presence of carbon tetrachloride. Looking at these structures reminds us that the chemical space can be endlessly large even with the combination of just two elements.

Rare C50 fullerene derivative C50Cl10
Page Top
Bromocarbons
The actual examples of C-Br compounds are much more limited, due to the large atomic radius of bromine (even larger than chlorine) and weaker C-Br bonds. Therefore, carbon tetrabromide (CBr4) and hexabromoethane (C2Br6) are the only known examples of perbromoalkane.
In organic synthesis, carbon tetrabromide is often used with triphenylphosphine to brominate primary alcohols under mild conditions. It is something that all synthetic chemists should remember as a useful reagent.
Aside from them, only a few compounds are known including tetrabromoethylene (C2Br4) and hexabromobenzene (C6Br6). The latter is sometimes used as a starting material of polycyclic aromatic hydrocarbons and liquid crystalline materials, since it can be functionalized in six directions by reactions such as cross coupling.
There are interesting halogenated carbon compound families such as perhaloallenes (X2C=C=CX2) and perhalobutatrienes (X2C=C=C=CX2). The last one that was synthesized among these was tetrabromobutatriene, which was synthesized in 2004 by adding bromine to dibromobutadiene (BrC≡C-C≡CBr) at low temperature.

Tetrabromobutatriene (Br2C=C=C=CBr2)
As for fullerene derivatives (C60Brn), examples with n=6, 8, 24 are known. In 2012, chlorinated and brominated versions of graphite were synthesized by treating graphite with liquid chlorine and bromine under microwave irradiation conditions. Besides the interest on their electronic properties, there have been growing expectations that these materials could be elaborated further to prepare graphene derivatives containing various functional groups.
Iodocarbons
Iodine has a particularly large atomic radius and therefore makes very limited numbers of compounds with carbon. Carbon tetraiodide (CI4) is the only periodoalkane ever known. Like the bromine counterpart, it is used for iodination reactions but is also known to decompose easily by the effect of light and heat.
Similar to bromine, C-I compounds such as tetraiodoethylene (C2I4), hexaiodobenzene (C6I6), tetraiodoallene (X2C=C=CX2), and tetraiodobutariene (X2C=C=C=CX2) are known. Under extremely highly pressurized conditions of about 35 GPa, hexaiodobenzene is known to undergo metallization, turning from an insulator into a good electric conductor while maintaining its molecular state. It is known to even become a superconductor at ultralow temperatures near 2 kelvins.
As we have seen, the chemical space composed of just the combination of carbon and halogens can be really deep. Next time, let us continue this line of thought and move on to the combinations of carbon-nitrogen, carbon-oxygen, and more.
Page Top
Introduction of the author
Kentaro Sato
[Brief career history] He was born in Ibaraki, Japan, in 1970. 1995 M. Sc. Graduate School of Science and Engineering, Tokyo Institute of Technology. 1995-2007 Researcher in a pharmaceutical company. 2008- Present Freelance science writer. 2009-2012 Project assistant professor of the graduate school of Science, the University of Tokyo. 2014-present Publicist for π-system figuration, scientific research on innovative areas.
[Specialty] Organic chemistry
Page Top





