Chemistry Chat
- Focusing on the Elements - Elements for the Fight against Cancer
The twentieth century saw an extraordinary extension of human life in industrialized countries. For example, the Japanese average longevity was around 44 years in 1900 and improved to over 80 years by 2000. The centenarian population was only 198 in 1965, but surpassed the 10,000 mark in 1998 and is now over 50,000 in 2012. In the next few decades, there may come a time when it isn’t rare to live longer than 100 years.
The advancement of medicine has been a big factor contributing to the dramatic extension of life. The discovery of antibiotics in particular was a revolutionary event, helping eradicate many of infectious diseases which had plagued mankind since the dawn of time. Also, our knowledge of taking care of our health such as controlling lifestyle diseases has advanced over the years along with the invention of many effective medicines.
The advancement of medicine in turn is largely attributed to that of chemical science, which studies the potentials of chemical elements and finds their most useful applications. No drug development can be separated from the science of chemical elements. In this column, let us look at some of the cancer drugs as examples to see what kind of roles chemical elements play in each case.
Platinum
As a cancer-fighting element, the first one that comes to one’s mind is probably platinum. Cisplatin, a platinum complex in which the central metal is coordinated with two ammonias and two chlorides, is a potent growth inhibitor of cancerous cells (the figure shown below). It is somewhat unusual that a purely inorganic complex like cisplatin is used as a medicine. It was discovered almost half a century ago, but it still has an important place in contemporary chemotherapy.
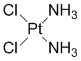
Cisplatin
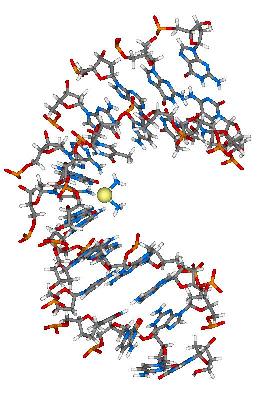
Cisplatin Binding to DNA
In 1965, an American chemist Barnett Rosenberg observed an unusually strong inhibition of the division of E. coli cells under certain conditions while he was studying the effects of electric field on the bacteria. To his surprise, the cause turned out to be not the electric field but the platinum electrodes used in the experiment. A trace concentration of platinum metal dissolved into the culture solution from the electrode and formed platinum complex, which reacted with the DNA of E. coli cells to interfere with their growth.
This effect was later demonstrated against cancer cells and cisplatin became a widely used drug for the treatment of mainly kidney, bladder, and ovarian cancers. There have also been structural analogues of cisplatin having modified ligands such as carboplatin and oxaliplatin, which are both important chemotherapeutics today.
Page Top
Fluorine
Fluorine is a common element in drug molecules, and one of the most famous F-containing drugs for cancer treatment is probably 5-fluorouracil (5-FU). Uracil is one of the nucleobases constituting human genes and 5-FU is its modified version in which one of the hydrogen atoms is replaced by a fluorine atom. This medicine works by sneaking into the biochemical process of nucleic acid and hinders DNA synthesis, thereby inhibiting cancer cell growth.
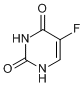
5-FU
In nucleic acid biosynthesis, uracil first reacts to form a chemical bond with a sugar molecule and then gets methylated at the 5-position to become thymidylic acid. 5-FU, being almost the same size as uracil, enters this biosynthetic pathway as a camouflaged uracil. However, because its 5-position is blocked by the fluorine atom the methylation cannot take place, resulting in the inhibition of the enzyme responsible for thymidylic acid synthesis and DNA biosynthesis is stopped consequently.
By the way, fluorine is of course the most electronegative element in all elements. Therefore, the introduction of fluorine into a drug candidate molecule in place of hydrogen can drastically alter the electronic property of the molecule. Also, compounds with low metabolic stability can be fluorinated at the metabolized position as a protection to improve their stability. For these reasons, fluorine has strengthened its presence in medicinal chemistry in recent years and this is also why the development of fluorination chemistry is progressing very fast.
Page Top
Arsenic
Arsenic is an element typically known to be toxic, but surprisingly it has medicinal application. The solution of arsenic trioxide (As2O3) is actually sold as anticancer drug! It was originally used in parts of China as a traditional medicine. When clinical trials were done using purified As2O3 to test its efficacy against different types of cancer, it was proven to be effective against a certain form of leukemia. It is now available in many countries.
It remains unclear exactly why arsenic trioxide, a supposed poison, possesses anticancer activity. But after all, all other anticancer drugs are similar in a way, in a sense that they are good medicine to our body by being poisonous to cancer cells. As the proverb goes, this is a good example of “fight poison with poison.”
Page Top
Boron
Boron (the “fifth element” in the periodic table) used to be an element rarely used in medicinal chemistry. The recent arrival of the boron-containing multiple myeloma drug Bortezomib (marketed as the name Vercade®), however, could change the tradition.
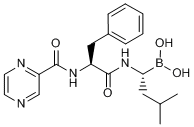
Bortezomib
Bortezomib is a dipeptide molecule in which the carboxylic acid on the C-terminus is replaced by boronic acid, and it targets the proteasome whose function is cleaning up of misfolded proteins. The boronic acid group of Bortezomib binds to the threonine residue of the proteasome’s active site to inhibit its function. The boron atom therefore plays a central role in the mechanism of action of this drug.
Many of common boron containing compounds are stable and have low toxicity. For chemists, boron is a very familiar element found in the reagent for hydroboration reaction or in the reactants of Suzuki-Miyaura reaction. It has never been a popular element in drug design, but its value possibly will change after the success of Bortezomib.
In another application, boron is used as a key component of BNCT (boron neutron capture therapy) research. In BNCT, boron is delivered to cancer cells and the cells are irradiated by neutrons. When boron-10 is hit by neutrons, it undergoes the nuclear fission reaction that gives lithium-7 and helium-4. The travel distance of these high energy particles is no more than a few nanometers long therefore they attack the cancer cells without damaging surrounding healthy cells. Because preferential delivery of the boron to the malignant cells is the key in BNCT, a variety of boron compounds are being developed.
Page Top
Elements Used in Radiation Therapy
Radiation therapy is an important cancer treatment along with chemotherapy and surgery. The most famous type is probably gamma ray irradiation by radioactive cobalt-60. Cobalt-60 is formed from cobalt-59, its stable isotope, by neutron activation in a nuclear reactor. Cobalt-60 emits a beta ray with the half-life of 5.27 years to become nickel-60, then emits two gamma rays which attacks the cancer cells.
The type of nuclei that can be used for radiation therapy is specified by the law and it includes a wide range of nuclei from tritium to radon-226. The list includes even artificial elements such as technetium and notorious ones of late such as iodine and cesium.
Ideally, radiation therapy targets only malignant cells and keeps healthy cells intact, but in reality simple irradiation cannot be completely cell-specific. Therefore, there are therapies in which a small radiation source is delivered to the affected tissue to treat it from inside.
The newest weapon of radiation therapy is a combination with antibody which works like a missile. For example, the adduct of a radioactive element such as yttrium-90 with an antibody was developed as a chemotherapeutic for lymphatic cancer. The drug utilizes the ability of antibodies to recognize the protein expressed on the surface of cancer cells, which reduces the chance of affecting untargeted areas of the body. Antibody technology is advancing fast and further development of this therapy in close future can be expected.
As we discussed so far, we humans employ all sorts of chemical elements to defend ourselves in the war against our biggest health threat, cancer. Because cancer is an ailment that has direct influence on our life and death, new medicines with even high risk of side effects tend to get government approval relatively easily. For this reason, a number of different approaches of drug development are being taken. Accordingly, we can look forward to seeing unusual elements utilized in future therapies based on innovative medical (and chemical) concepts.
Page Top
Introduction of the author
Kentaro Sato
[Brief career history] He was born in Ibaraki, Japan, in 1970. 1995 M. Sc. Graduate School of Science and Engineering, Tokyo Institute of Technology. 1995-2007 Researcher in a pharmaceutical company. 2008- Present Freelance science writer. 2009-2012 Project assistant professor of the graduate school of Science, the University of Tokyo.
[Specialty] Organic chemistry
Page Top





