Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Chemistry Chat
- Focusing on the Elements - Molecular Regular Polygons
Kentaro Sato
Regular Triangles
Cyclopropane units are sometimes found as part of the structures of natural products such as terpenes. Among them, the two compounds that stand out are FR900848 and U-106305, both of which were discovered by pharmaceutical companies as drug candidates. As you can see, they feature a uniquely repetitive array of cyclopropane units. It is interesting to note that these two compounds that share this distinctive substructure were isolated from completely different organisms and also have completely different biological activities.
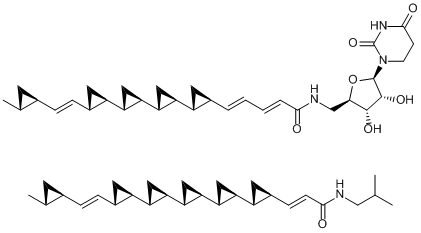
FR900848 (top), U-106305 (bottom)
Squares
However, cyclobutadiene can be the ligand of organometallic complexes with metals such as iron. In these cases, the four-membered ring accepts a pair of electrons from the metal to complete the 6π aromatic system. In the iron complexes, it is known that all of the carbon-carbon bonds become equivalent and cyclobutadiene coordinates as a square.
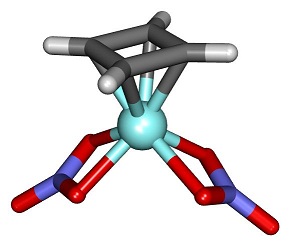
An example of cyclobutadiene organometallic complex
In 2013, a compound called pyramidane was synthesized by V. Y. Lee and Akira Sekiguchi and the structure was determined by X-ray analysis. The molecule’s shape is literally pyramidal, with its structure composed of germanium or tin atom positioned at the apex and the four carbon atoms forming the square base. Each of the four carbon atoms is bonded to a trimethylsilyl group that protects the sensitive complex against decomposition. What an absolutely amazing creation!
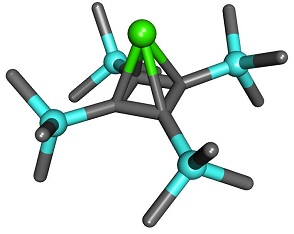
Pyramidane
Regular Pentagons
As for equilateral five-membered ring compounds, cyclopentadienyl anion is well-known. The cyclopentadienyl anion formed by the deprotonation of cyclopentadiene is a 6π electronic system that shows aromatic character. The five carbon atoms are equivalent and the molecule has a shape of regular pentagon. When it binds to transition metals, the resulting compounds are generally called metallocenes. Metallocenes are familiar compounds to us today, but their early discoveries were so impactful that they triggered the emergence of a new genre called organometallic chemistry.
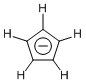
Cyclopentadienyl anion
Regular Hexagons
As mentioned in the beginning, benzene is a symbolic compound possessing regular hexagonal structure. There are a number of compounds in which several benzene rings are fused to form a larger hexagonal structure as a whole. The compound consisting of seven fused benzene rings is called coronene after the corona of the Sun.
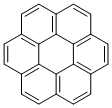
Coronene
Hexabenzocoronene, which is coronene plus six additional fused benzene units, is considered a trendy material in recent nanotechnology research. Kekulene, which you could call “the benzene made of benzenes,” was synthesized in 1978 for the first time. Needless to say, this macrocyclic aromatic compound was named after August Kekulé, the German chemist who proposed the six-memebered ring structure of benzene.

Hexabenzocoronene (Left), Kekulene (Right)
Regular Heptagons
We have so far seen four-, five-, and six-membered ring examples with 6π electronic system, so let us move on to analogous seven-membered rings. The chemical species called tropylium cation (C7H7+) is the most famous one, which is synthesized from cycloheptatriene (by Lewis acidic treatment, for example).
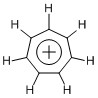
Tropylium cation
Let us look through compounds of the slightly different family. As shown below, oxo-carbon acids with the ring sizes from three to seven are known. Upon releasing two protons, these compounds become regular polygonal structures by delocalizing the charges. Because the dianions are stabilized by aromaticity, these oxo-acids show exceptionally strong acidities for compounds only made of C, H, and O.

Oxo-carbon acids
Regular Octagons
Cyclooctatetraene ([8]annulene) does not assume flat conformation unlike benzene and related aromatic compounds. The flat structure would be an 8π antiaromatic system, therefore the boat-shape becomes a more stable structure. However, the dianion formed by treatment with reducing agents is a 10π aromatic system that can exist as a flat regular octagon. Also, in some organometallic complexes containing cyclooctatetraene, the ligand is known to coordinate with the metal center as a flat regular octagon.
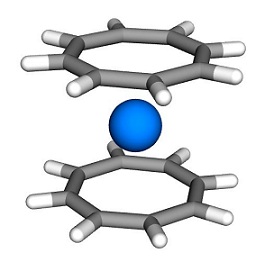
Uranocene
In 2006, a neutral compound containing equilateral octagonal structure was synthesized. This compound composed of eight fused thiophene units looked like sunflower, so it was named “sulflower” by combining sulfur and flower.
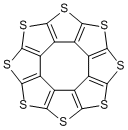
Sulflower
Regular Decagons and Beyond
For instance, there is a structure called [10.5]coronene, which consists of five-membered rings positioned on each side of a decagon. This structure has been of great theoretical interest for a long time but its synthesis has yet to be achieved.
![[10.5]Coronene](/assets/cms-images/162-yomo1_11.gif)
[10.5]Coronene
One could also imagine a structure like the one shown below, which has six- and four-membered rings placed alternately on the sides of a dodecagon. This type of compounds composed of fused benzene and cyclopentadiene rings (known as [N]phenylenes) are widely known, but this particular compound has never been synthesized. It has been difficult because both the inner and outer rings (12π and 24π, respectively) are antiaromatic. For this reason, this molecule has a fitting nickname “antikekulene.”
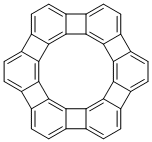
Antikekulene
Introduction of the author
Kentaro Sato
[Specialty] Organic chemistry

