Maximum quantity allowed is 999
请选择数量
CAS RN: 64443-05-6 | 产品编码: T2665
Tetrakis(acetonitrile)copper(I) Hexafluorophosphate
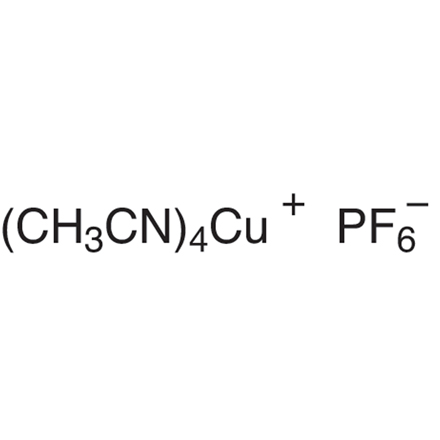
技术规格
| Appearance | White to Almost white powder to crystal |
| Purity(Potassium permanganate method) | min. 97.0 % |
| NMR | confirm to structure |
物性(参考值)
| 熔点 | 160 °C(dec.) |
GHS
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相关法规
运输信息
| HS编码* | 2931.59-000 |
应用
Perfluoroalkylation using Perfluoro Acid Anhydrides

Reference
- Perfluoroalkylation of Unactivated Alkenes with Acid Anhydrides as the Perfluoroalkyl Source
应用
Copper Catalyzed Asymmetric Conjugate Addition
References
- Catalytic Asymmetric Synthesis of Chiral Tertiary Organoboronic Esters through Conjugate Boration of β-Substituted Cyclic Enones
- Searching for Practically Useful P-Chirogenic Phosphine Ligands
应用
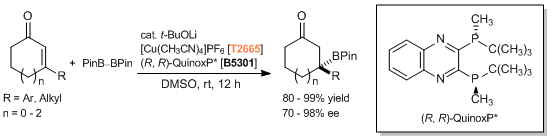
Catalytic Asymmetric Vinylogous Conjugate Addition of Unsaturated Butyrolactones to α,β-Unsaturated Thioamides
Reference
应用
Copper-catalyzed C―H Amidation of Unactivated Arenes

Typical Procedure:
To a stirred mixture of [Cu(CH3CN)4]PF6 (0.010 g, 0.026 mmol) and neocuproine (0.005 g, 0.024 mmol) in the arene (ca. 1 mL) is added N-tosyloxy-2,2,2-trichloroethylcarbamate (0.050 g, 0.138 mmol) and the mixture is allowed to stir at 140 °C for 8 h. The reaction mixture is cooled to room temperature, filtered through a pad of silica, washed with CH2Cl2, and the filtrate is concentrated under vacuum. The residue is purified by silica chromatography using 5–15% Et2O in hexanes to obtain the regioisomeric mixture of aminated arenes. The isomer ratio is determined by NMR using 1,3,5-trimethoxybenzene as a reference.
To a stirred mixture of [Cu(CH3CN)4]PF6 (0.010 g, 0.026 mmol) and neocuproine (0.005 g, 0.024 mmol) in the arene (ca. 1 mL) is added N-tosyloxy-2,2,2-trichloroethylcarbamate (0.050 g, 0.138 mmol) and the mixture is allowed to stir at 140 °C for 8 h. The reaction mixture is cooled to room temperature, filtered through a pad of silica, washed with CH2Cl2, and the filtrate is concentrated under vacuum. The residue is purified by silica chromatography using 5–15% Et2O in hexanes to obtain the regioisomeric mixture of aminated arenes. The isomer ratio is determined by NMR using 1,3,5-trimethoxybenzene as a reference.
References
参考文献
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






