Chemistry Chat
- Focusing on the Elements - Compounds Composed of Two Elements (3)
Compounds Composed of Carbon and Sulfur
This column covered compounds composed of just carbon and oxygen previously. This time, let us start off with the combination of carbon and sulfur, the element positioned directly below oxygen in the periodic table.
From this combination, one would probably think of carbon disulfide (CS2) first. Carbon disulfide is isoelectronic to carbon dioxide, but unlike CO2 this compound exists as a liquid at room temperature, melting at -111 °C and boiling at 46 °C. Although you might expect it to have an unpleasant smell characteristic of sulfur compounds, the smell actually originates from its decomposition impurities and pure CS2 is supposed to have an ether-like odor.
CS2 has been used as a solvent for the manufacture of rubber and cellophane products. However, it is being used less and less frequently these days due to its high toxicity. It is a neurotoxin that is even an ingredient of some insecticides, so it has to be used with appropriate caution.
Carbon monosulfide (CS), the compound isoelectronic to carbon monoxide, is also known. It has been detected in outer space and is also formed by the decomposition of carbon disulfide mediated by light or electric discharge. Monomeric CS, however, is extremely unstable and easily polymerize to form (CS)n.
More than 10 other examples of low molecular weight compounds composed of just carbon and sulfur are known, some of which are shown below. Since organic sulfur compounds often exhibit unique electronic properties and constitute an actively studied field, the list is only expected to grow.

Organic sulfur compounds
In 2006, a new member of carbon sulfide family entered the scene boasting a beautiful, unprecedented structure. The structure is shown below and the compound was named Sulflower after sulfur and flower. Aside from functioning as an organic semiconductor, it has been suggested computationally that it may have hydrogen storage ability by adsorbing hydrogen molecules within its crystal structure, which is a remarkable potential.
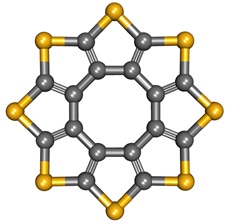
Sulflower
As you can see, Sulflower has a structure composed of 8 thiophene units fused in circular fashion. According to the theoretical calculation, the molecule is planar when the number of thiophene units is 8 or 9. The molecule takes a crown-like shape and a saddle-like shape when the thiophene units are less than 8 and more than 9, respectively. It will be exciting to learn about the properties of these non-planar Sulflowers, as well as those of variants containing other heteroatoms, that could potentially be useful materials.
Page Top
Compounds Composed of Carbon and Nitrogen
(1) Cyano Compounds
Nitrogen sits on the right side of carbon in the periodic table and is an element found widely in a number of natural organic compounds such as amino acids and alkaloids. Like hydrogen and oxygen are, nitrogen is a great partner of carbon in organic chemistry. Yet, compounds composed of only carbon and nitrogen might be comparatively harder to imagine.
The simplest example is cyanogen, which has a molecular formula of (CN)2 and was first synthesized in 1815 by the renowned French chemist Joseph Gay-Lussac. It is a gas with a pungent odor and is highly toxic similar to other cyano compounds.
When Halley’s Comet approached the earth in 1910, this cyanogen was detected spectroscopically in the tail of the comet. Since it was expected that the earth would pass through the tail of the comet, it spread a false rumor that the poisonous gas would exterminate all life forms on the earth, creating a wave of panic around the world. But of course, the concentration of the gas in the tail of the comet turned out to be so dilute that nothing happened to the earth.
Dicyanoacetylene (N≡C–C≡C–C≡N) is obtained by passing nitrogen gas through graphite at around 3000 K. It is a clear and colorless liquid that is known to burn with the hottest flame among all substances (ca. 5260 K).
In outer space, it is known that compounds called cyanopolyynes (H–(C≡C)n–C≡N) exist. Dicyanoacetylene is also considered to exist with them, but it has no rotational spectrum because of its molecular symmetry, therefore there is no effective way to detect it. It is known to exist in the atmosphere of Titan, the satellite of Saturn, so chances are that it could be a building block of other complex molecules.
In this way, one could imagine compounds based on only carbon and nitrogen by replacing the hydrongen atoms of hydrocarbons with cyano groups. Compounds such as tetracyanomethane (C(CN)4) and hexacyanobenezene (C6(CN)6) have been actually synthesized. Among them, the most famous one is probably tetracyanoethylene (TCNE). With the four electron-withdrawing cyano groups attached on a small carbon scaffold, this molecule serves as an excellent electron acceptor in the research of organic semiconductors and organic superconductors.
Page Top
(2) Azide Compounds
Metal complexes containing multiple azide ligands have been synthesized in recent years. Tetraazidomethane (C(N3)4), which has four azide groups bonded to one carbon atom, was also synthesized surprisingly recently in 2007. After many attempts, the only successful way to prepare it has been the reaction between trichloroacetonitrile (Cl3CCN) and sodium azide (NaN3). The ratio of carbon to nitrogen is 1 to 12 for this molecule, which is somewhat astonishing.

Tetraazidomethane
As is well known, azide compounds have explosive properties and their handling requires great care. Tetraazidomethane, with the four azide groups crowded within a small molecule, is something of an ultimate high-energy substance and extremely dangerous. The author of the report says that, “Tetraazidomethane is extremely dangerous as a pure substance. It can explode at any time—without a recognizable cause. Less than a drop of this compound isolated by gas chromatography is able to destroy completely not only the glass trap but also the vacuum Dewar flask of the cooling bath.” Personally, I would never like to try this experiment regardless of the quality of lab environment!
Page Top
(3) Azafullerenes
If the compound having the highest N to C ratio is tetraazidomethane, then the compound with very high C to N ratios would be these azafullerenes. Azafullerenes have been synthesized from C60 fullerene in multiple steps by incorporating nitrogen atom(s) into the original skeleton to produce such compounds as C59N+ cation and the dimeric (C59N)2, in which two buckyballs are connected at the carbons adjacent to the nitrogens. There is also a report of the synthesis of C69N+ cation starting from C70.
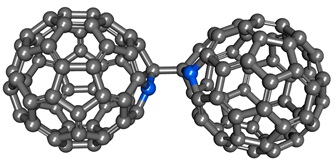
(C59N)2
In addition to these examples, the molecule of fullerene in which a nitrogen atom is trapped inside the fullerene structure (N@C60) has been isolated. This “endohedral fullerene” compound was synthesized by irradiating ordinary fullerene with nitrogen plasma, which enables the “implantation” of nitrogen. Various derivatives have been actively synthesized in recent years and they could be developed into useful applications.
Page Top
(4) Polymeric Carbon Nitrides
“Photocatalysts” which are capable of decomposing substances such as water with the assist of light have been applied to various areas ranging from environmental purification to energy creation, and are one of the innovations that emerged from Japan. Whereas the development of this field has centered on inorganic materials such as titanium dioxide, recent years have seen the advent of organic materials with promising properties. One of them is called graphitic carbon nitride (g-C3N4).
Graphitic carbon nitrides are obtained by heating nitrogen-rich compounds such as melamine, and by depositing a small amount of co-catalysts (e.g. platinum), these materials can decompose water under visible light. And more recently, synthetic improvement has led to the introduction of a new version of g-C3N4 having a larger surface area, which enables more efficient decomposition of harmful substances such as nitrogen monoxide. These polymers can certainly be counted as one of the promising groups of carbon materials.
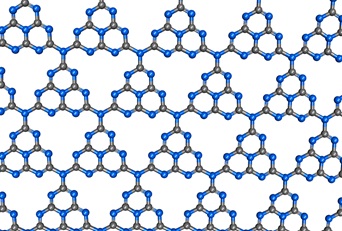
Graphitic carbon nitride
Furthermore, there is a substance called “hexagonal crystalline carbon nitride” (β-C3N4), which has a structure consisting of three-dimensional network of carbon and nitrogen and is predicted to be harder than diamond. At present, only nano-scale crystal preparation has been successful, but it sure would have a wide range of applications if it could be prepared on practical scales.
The chemical space of carbon nitrides can be just as deep as other substances, as it encompasses explosives, organic electronic devices, and super-hard materials. There seems to be a room for further developments and discoveries of fascinating compounds.
Page Top
Introduction of the author
Kentaro Sato
[Brief career history] He was born in Ibaraki, Japan, in 1970. 1995 M. Sc. Graduate School of Science and Engineering, Tokyo Institute of Technology. 1995-2007 Researcher in a pharmaceutical company. 2008- Present Freelance science writer. 2009-2012 Project assistant professor of the graduate school of Science, the University of Tokyo. 2014-present Publicist for π-system figuration, scientific research on innovative areas.
[Specialty] Organic chemistry
Page Top




