Chemistry Chat
- Focusing on the Elements - New Allotropes of Main Group Elements
When I was a high schooler, I was taught that there were only three allotropes of carbon: diamond, graphite, and amorphous carbon. But after the 80's, textbooks were rewritten drastically with the addition of buckyball fullerene C60, cylindrical carbon nanotube, and single-layered graphene. It should need no explanation now as to how much these new carbon allotropes have contributed to the advancement of diverse fields including materials science, organic chemistry, and condensed matter physics.
If carbon offers such interesting variations of substances, it should be natural for us to wonder, what about other elements? In fact, new allotropes of other elements, with structures ranging from spherical to planar to three-dimensional networks, have been reported one after another in recent years to push the envelope of science. In this article, let us go through some of the new allotropes of main group elements.
Boron
Boron is positioned on the left side of carbon in the periodic table and known to take some unique cluster structures because of its electron deficiency and the property to form three-center two-electron bonds. A number of boron clusters of the BnHn2− type that take deltahedral structures (polyhedral structures consisting of equilateral triangular sides), are known. Dodecahydrododecaborate ion (B12H122−), for example, has a beautiful icosahedral structure.
Many of the allotropes of boron form larger network assemblies consisting of this B12 icosahedral cluster. For example, α-rhombohedral boron has a nanostructure in which the icosahedral clusters are packed in a similar way to the arrangement called “cubic close packing.” In addition, other structural variations are known such as amorphous boron and “face-centered cubic” metal form of boron (which exists only under high pressure conditions).
Then, is it possible for boron to form spherical caged structures like fullerene? Although the exact boron analogue of C60 may be too difficult, B80, which has boron atoms added to the center of each hexagonal side, was considered stable enough to exist and computational studies have been done.
More recently, however, the B40 cluster was discovered (Nat. Chem. 2014, 6, 727.). The formation of this all-boron fullerene was confirmed by Lai-Sheng Wang's group at Brown University, who treated boron with laser irradiation and cooled with helium gas.
Named “borospherene”, this molecule consists of 48 triangles, 2 six-membered rings, and 4 seven-membered rings, all based on boron atoms. As shown in the figure below, it belongs to the D2d point group. It is somewhat hard to picture and really makes you wonder why this shape is so stable. It is possible that other borospherenes with different number of boron atoms and structure will be discovered in near future.
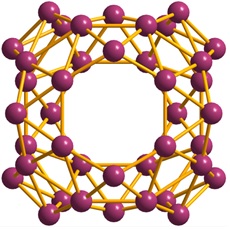
Borospherene (extracted from Wikipedia)
Separately from borospherene, the Wang group has considered a boron-based sheet-like structure and named it “borophene” from its relation to graphene. The structure of borophene contains a planar six-membered ring surrounded by a network of triangles. The Wang group has shown evidence for the formation of this B36 cluster, which is considered as a basic unit (Nat. Commun. 2013, 5, 3113.). The science of boron, like carbon did, could develop further to expand the fascinating world of “nano-borons.”
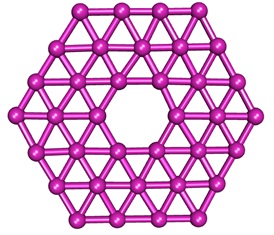
Borophene
Page Top
Nitrogen
Let us move on to nitrogen, the other neighbor of carbon. Neutral allotropes of nitrogen had long been unknown other than normal N2 gas, but the existence of tetranitrogen (N4) was confirmed in 2002 (Science 2002, 295, 480.). However, its lifetime was only microseconds long and it was too unstable to be isolated. The fragmentation pattern suggested that the structure of tetranitrogen was linear and formed by the weak interaction of two N2 molecules.
Besides that, the nitrogen analogues of benzene (hexazine, N6), cubane (N8), and fullerene (N60) have been investigated theoretically even though they have not been synthesized actually. These compounds, if they can be prepared, are expected to release high energies upon decomposition and thus have a potential application as explosive materials.
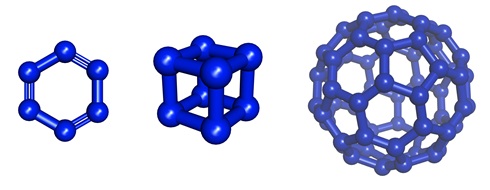
Theoretically considered nitrogen allotropes
In 2004, Max Planck Institute for Chemistry in Germany reported the synthesis of “polynitrogen” (Nat. Mater. 2004, 3, 558.). In this work, it was found that nitrogen atoms form a single-bonded polymeric network when compressed under the conditions of 110 GPa and 2000 K.
This polynitrogen is thought to possess five times or even higher energy capacity than the most powerful nonnuclear explosives known today. Explosives of this kind have appeared in science fictions in the past, but they are becoming a reality. Of course, this polymeric allotrope of nitrogen can be neither prepared practically nor stored, so it has no prospect of being used as a weapon.
As for solely nitrogen-based ions, azide ion (N3−) is known for a long time. Its handling requires caution because of its explosive property, but it has a wide application including preservative and detonating agent. In organic synthesis, it is a useful regent used traditionally as a source of nitrogen.
There is also an ion called pentazolyl anion (N5−), which is an all-nitrogen pentagonal ion isoelectric to cyclopentadienyl anion. It was obtained by [3+2] cycloaddition of p-methoxybenzenediazonium cation and azide that formed the pentazole skeleton, followed by oxidative deprotection using cerium ammonium nitrate (Chem. Comm. 2003, 1016.). Even though the anion is stabilized to an extent by the aromaticity, the repulsions between the nitrogen atoms cause it to decompose with the half-life of 2.2 days.
As another example, there is a species called pentazenium cation (N5+) (J. Am. Chem. Soc. 2001, 123, 6308.). This substance was discovered by the High Energy Density Matter program run by the U.S. Air Force.
Pentazenium was prepared by reacting fluorodiazonium (N2F+) and azide. The SbF6− salt of pentazenium was stable enough for isolation and X-ray crystallographic analysis, and the cation was found to have a V-shaped bent structure.
In theory, this pentazenium cation and azide or pentazolyl anion would form a salt composed of only nitrogen atoms. But who would dare to try to synthesize it, considering its easily predictable explosive nature? Well, it turns out that similar experiments have been done and salts like N5+[B(N3)4]− and N5+[P(N3)6]− have been actually synthesized (Angew. Chem. Int. Ed. 2004, 43, 4919.)! This paper has a picture of an exploded Teflon tube and the experimental section is filled with cautionary remarks, describing an extreme case of chemical research.
Page Top
Oxygen
A well-known allotrope of oxygen is ozone (O3). It is a pale blue toxic gas and its strong oxidizing property is second only to fluorine. Ozone is produced by ultraviolet irradiation or silent discharge of oxygen, and in synthetic laboratories, it is used to oxidatively cleave carbon-carbon double bonds in the reaction known as ozonolysis. In some areas, it is used for disinfection of tap water.
The presence of tetraoxygen, the quartet of oxygen atoms, was predicted in 1924 by Gilbert Lewis, who is famous for formulating the definition of acids and bases. The experimental demonstration, however, proved difficult and had been elusive until 2001, when the group at University of Rome finally detected it by mass spectrometry. The structure of tetraoxygen was neither four-membered ring nor Y-shaped as anticipated, but turned out to be a complex of two oxygen molecules, one in ground state and the other in excited state.
There is one more allotrope of oxygen. When oxygen is compressed with gradually increased pressure at room temperature, the volume decreases steeply after 10 GPa and the color changes from blue to red. The identity of this so-called “red oxygen” had long been a mystery, but it was elucidated in 2006 to be the O8 cluster composed of four oxygen molecules based on the powder X-ray diffraction patterns (Phys. Rev. Lett. 2006, 97, 085503.). The discovery came as a major surprise as this “octaoxygen” structure had not been predicted even theoretically.
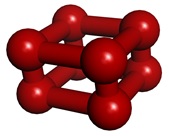
Unit structure of ε-oxygen (“red oxygen”)
Page Top
The examples we just covered suggest that the chemistry of allotropes has more unexplored possibilities. Next time, let us cover allotropes of the period 3 elements and more.
Page Top
Introduction of the author
Kentaro Sato
[Brief career history] He was born in Ibaraki, Japan, in 1970. 1995 M. Sc. Graduate School of Science and Engineering, Tokyo Institute of Technology. 1995-2007 Researcher in a pharmaceutical company. 2008- Present Freelance science writer. 2009-2012 Project assistant professor of the graduate school of Science, the University of Tokyo. 2014-present Publicist for π-system figuration, scientific research on innovative areas.
[Specialty] Organic chemistry
Page Top



