Maximum quantity allowed is 999

The term regenerative medicine refers to a next-generational field of medical techniques in which cells are taken from patients, cultured, expanded, and returned to the patient in some manner in order to regenerate tissues or promote wound healing. The vast majority of these techniques, including cell sheet engineering – using patient-derived cell sheets to promote wound healing, and decellularized whole-organ engineering – the generation of intact organs using a decellularized scaffold along with the patient’s own cells, involve the use of stem cells with the same genotype as the original patient. Not only does this allow for the avoidance of transplant rejection, but it is also widely expected to help overcome the worldwide organ shortage.
The type of stem cell perhaps most frequently employed in these applications are known as induced pluripotent stem cells (iPSCs). iPSCS were developed by Professor Shinya Yamanaka at Kyoto University in a landmark paper in 2006.1) Professor Yamanaka was able to return fibroblasts to an undifferentiated state and confer upon them the same regenerative potential as embryonic stem cells through the induction of a combination of four transcription factors – Oct3/4, Sox2, Klf4, and c-Myc. He also demonstrated how these cells could be coerced down different lineages through the application of appropriately timed signaling factors.

Reprogamming methods used in the production of iPS cells
In recent years, organoids have come to the forefront as a model for the application of human iPSCs in regenerative medicine. Organoids are in-vitro 3D cultured cell aggregates derivable from both iPSCs as well as somatic stem cells which are not only capable of self-organization and long term self-renewal, but which also exhibit similar morphology and function to the tissues from which they were derived.2) This is achieved through the use of physical and biochemical cues, such as extracellular matrix components and niche factors, which are able to recapitulate cells' natural environment within living tissue.

Organoid culture pipeline
Here at TCI, we have an extensive lineup of chemicals frequently used in both organoid culture and iPS generation / differentiation. Find below a table containing common medium additives used to generate tissue-specific organoids from both hSSCs and hiPSC, as well as several lists of common inhibitors and activators, organized by signaling pathway.
| Tissue Type | Origin | Culture Conditions | Reference |
|---|---|---|---|
| Small Intestine | hAdSCs | A83-01, Nicotinamide, N-Acetylcysteine, Y-27623, SB202190, EGF, Rspondin1, Noggin, Wnt-3a | 3 |
| hPSCs | EGF, Rspondin1, FGF-4, Noggin, Wnt-3a, Activin A | 4 | |
| Large Intestine | hAdSCs | A83-01, Nicotinamide, N-Acetylcysteine, Y-27623, SB202190, EGF, Rspondin1, Noggin, Wnt-3a | 3 |
| Stomach | hAdSCs | A83-01, Nicotinamide, N-Acetylcysteine, Y-27623, EGF, Rspondin, FGF-10, Gastrin, Noggin, Wnt-3a | 5 |
| hPSCs | CHIR 99021, Y-27623, Retinoic Acid, EGF, FGF-4, BMP-4, Noggin, Activin A, Wnt-3a | 6 | |
| Lung | hAdSCs | A83-01, Nicotinamide, Y-27632, SB202190, Rspondin1, FGF-7, FGF-10, Noggin | 7 |
| hPSCs | [for Differentiation] SB431542, SANT-2, SU-5402, bFGF, Noggin, SHH, SAG, Activin A [for Organoid Culture] CHIR 99021, SB431542, FGF-4, Noggin | 8 | |
| Brain | hPSCs | Y-27632, Heparin, 2-Mercaptoethanol, bFGF, Insulin | 9 |
| Liver | hAdSCs | [for Organoid Culture] Y-27632, A83-01, Nicotinamide, N-Acetylcysteine, Forskolin, EGF, Rspondin1, FGF-10, Gastrin, Noggin, Wnt-3a, HGF [for Hepatocyte Differentiation] A83-01, DAPT, Dexamethasone, EGF, FGF-19, BMP-7, Gastrin, HGF | 10 |
| hPCSs | bFGF, BMP-4, Activin A, HGF, Oncostatin M | 11 | |
| Pancreas | hAdSCs | A83-01, Nicotinamide, N-Acetylcysteine, EGF, Rspondin1, FGF-10, Gastrin, Noggin, Wnt-3a | 12 |
| Kidney | hPCs | [for Differentiation] CHIR 99021, 2-Mercaptoethanol, Heparin, Retinoic Acid, 1-Thioglycerol, bFGF, FGF-9, BMP-2, BMP-4, Insulin, Activin A, Holo-transferrin [for Organoid Culture] CHIR 99021, Heparin, FGF-9, HGF, GDNF | 13, 14 |
| Prostate | hAdSCs | Y-27623, A83-01, Nicotinamide, N-Acetylcysteine, SB202190, Prostaglandin E2, Testosterone, EGF, Rspondin1, FGF-10, bFGF, Noggin | 15 |
| hPCs | [for Differentiation] FGF-10, Activin A, Wnt-10b [for Organoid Culture] Retinoic Acid, Testosterone, EGF, Rspondin1, Noggin | 16 |
References
- 1) Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors
- 2) Engineering organoids
- 3) Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett's Epithelium
- 4) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro
- 5) In Vitro Expansion of Human Gastric Epithelial Stem Cells and Their Responses to Bacterial Infection
- 6) Organoid Models of Human and Mouse Ductal Pancreatic Cancer
- 7) Long‐term expanding human airway organoids for disease modeling
- 8) In vitro generation of human pluripotent stem cell derived lung organoids
- 9) Generation of cerebral organoids from human pluripotent stem cells
- 10) Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver
- 11) Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells
- 12) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids
- 13) Generation of kidney organoids from human pluripotent stem cells
- 14) Engineered Kidney Tubules for Modeling Patient-Specific Diseases and Drug Discovery
- 15) Organoid culture systems for prostate epithelial and cancer tissue
- 16) Directed Differentiation of Human Embryonic Stem Cells into Prostate Organoids In Vitro and its Perturbation by Low-Dose Bisphenol A Exposure
Growth Factors
Characterized as cytokines, growth factors are the name for soluble proteins that initiate signaling cascades in cells related to proliferation, differentiation, survival, inflammation, and tissue repair.
| Chemical Name | Target | Effect |
|---|---|---|
| rhEGF | EGFR | Ligand/Agonist |
| rhFGF2 | FGFR | Ligand/Agonist |
Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways
- Mechanisms underlying differential responses to FGF signaling
Prostaglandin Signaling Pathway
Prostaglandins, a metabolic derivative of arachidonic acid, play key roles in vasodilation and the generation of the inflammatory response.
| Chemical Name | Target | Effect |
|---|---|---|
| Prostaglandin E1 | EP1–4 | Ligand/Agonist |
| Prostaglandin E2 | EP1–4 | Ligand/Agonist |
Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation
MAPK Signaling Pathways
The mammalian MAPK (Mitogen-Activated Protein Kinase) signaling pathways transmit a wide variety of signals from outside the cell through the activation of MAPKs, and are divided into three subgroups based on the specific MAPK at work: ERK, JNK, or p38.
ERK1/2 Signaling Pathway
The ERK/MAPK signaling pathway, playing major roles in cell proliferation and differentiation, begins via extracellular signals received at membrane-embedded receptor proteins such as receptor tyrosine kinases, integrins, and ion channels. Different combinations of ligand/receptor result in the activation of slightly different downstream effectors, but in general, signals from the receptor first reach an adaptor protein such as Shc, GRB2, or Crk, which is then transmitted via activation of a guanine nucleotide exchange factor such as SOS or C3G. This in turn allows for the activation of GTP binding proteins such as Ras and Rap1, which phosphorylate and activate the MAPKKK (MAPK Kinase Kinase) Raf, which phosphorylates and activates the MAPKK (MAPK Kinase) MEK1/2, which finally phosphorylates and activates ERK. Activated ERK dimer is then able to phosphorylate and activate downstream molecules not only in the cytoplasm but also in the nucleus.

p38 Signaling Pathway
As one of the three principal MAPK signaling pathways in mammals, the p38 MAPK signaling pathway plays a similar role as the JNK signaling pathway as a mediator of the cell’s response to environmental and genetic stress. In mammals, four isoforms exist (the p38α, p38β, p38γ, and p38δ isoforms), with p38α/β as the main isoforms. Upon direct and indirect activation via Akt, TNFα, Wip1, etc., p38α/β is able to phosphorylate and activate various targets in both the cytoplasm and nucleus, the most prominent of which being p53, MSK1/2, and HBP1. p38α/β activation also results in the downregulation of certain effector molecules such as Cdc25B and CycD1, highlighting the role that p38 plays in the cell cycle.
JNK Signaling Pathway
As one of the three principal MAPK signaling pathways in mammals, the JNK MAPK signaling pathway plays a similar role as the p38 signaling pathway as a mediator of the cell’s response to environmental and genetic stress. Upon direct and indirect activation via Akt, Tak1, TNFα, ROS, etc., JNK is able to phosphorylate and activate various targets in both the cytoplasm and nucleus, the most prominent of which being p53, PPARγ, HSP1, c-Jun, and Stat3. JNK activation also results in the downregulation of certain effector molecules such as Bcl2 and Bim, highlighting the role that JNK plays in determination of cell fate.

| Chemical Name | Target | Effect |
|---|---|---|
| GW-5074 | c-Raf | Inhibitor |
| Sorafenib | c-Raf, B-Raf | Inhibitor |
| PD 98059 | MEK1/2, AHR | Inhibitor |
| SL 327 | MEK1/2 | Inhibitor |
| PD 184352 | MEK1/2 | Inhibitor |
| SP 600125 | JNK1/2/3, Aurora A, FLT3, TRKA | Inhibitor |
| SU 3327 | JNK | Inhibitor |
| SB 239063 | p38α/β | Inhibitor |
| SB 203580 | p38 | Inhibitor |
| FR 180204 | ERK1/2 | Inhibitor |
| Honokiol | ERK1/2, Akt | ERK1/2: Activator, Akt: Inhibitor |
| PD 169316 | p38 | Inhibitor |
| VX-702 | p38α | Inhibitor |
Products
- G0609
- GW-5074
- O0599
- Sorafenib
- A2529
- PD 98059
- L0331
- SL 327
- P2174
- PD 184352
- A2548
- SP 600125
- A2940
- SU 3327
- B5898
- SB 239063
- F0864
- SB 203580
- F1214
- FR 180204
- H1309
- Honokiol
- P2532
- PD 169316
- V0147
- VX-702
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer
- The p38 Pathway: From Biology to Cancer Therapy
- The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer
PI3K/Akt Signaling Pathway
PI3K mediates conversion of PIP2 to PIP3 on the inner leaflet of the cell membrane upon recruitment to the membrane following activation of various receptor proteins including integrin, RTKs, cytokine receptors, B-cell receptors, and GPCRs. PIP3 acts as binding sites for various factors such as PDK1 and mTORC2, as well as Akt, which is activated via phosphorylation by PDK1 and mTORC2. Akt (Protein Kinase B) is a protein kinase which has as target proteins mTORC1, MDM2, Bad, CDK2, Lamin A, IKKα, FOXO1, P27, and GSK-3, among others, giving it an important role in the regulation of such cellular processes as cell growth, survival, motility, metabolism, and protein synthesis.

| Chemical Name | Target | Effect |
|---|---|---|
| LY 294002 | PI3K | Inhibitor |
| Miltefosine | PI3K | Inhibitor |
| 3-Methyladenine | PI3K | Inhibitor |
| Quercetin | PI3K | Inhibitor |
| Wortmannin | PI3K | Inhibitor |
Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function
- Targeting PI3K/Akt signal transduction for cancer therapy
PKC Signaling Pathway
Protein Kinase C (PKC) is a family of serine/threonine-kinases divided into three subfamilies based on which associated factors (Ca2+, DAG) are required for their phosphorylation-mediated activation.
| Chemical Name | Target | Effect |
|---|---|---|
| Bisindolylmaleimide I | PKC | Inhibitor |
| Fasudil | PKC, etc. | Inhibitor |
| Staurosporine | PKC, etc. | Inhibitor |
Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Protein kinase C signaling and oxidative stress
Wnt Signaling Pathway
The Wnt signaling pathway can be divided into the canonical and non-canonical (planar cell polarity and Wnt-calcium) pathways. In the canonical pathway, the Wnt receptor (a dimer of Fz and LRP5/6) sequesters the β-catenin destruction complex upon ligand binding, allowing the build-up of β-catenin in the nucleus. Once in the nucleus, β-catenin complexes with Tcf/Lef transcription factors to control the expression of various downstream genes. The canonical pathway plays major roles in the determination of cell fate during embryonic development, including the determination of body axis, and contributes to the regulation of differentiation and maintenance of stemness.
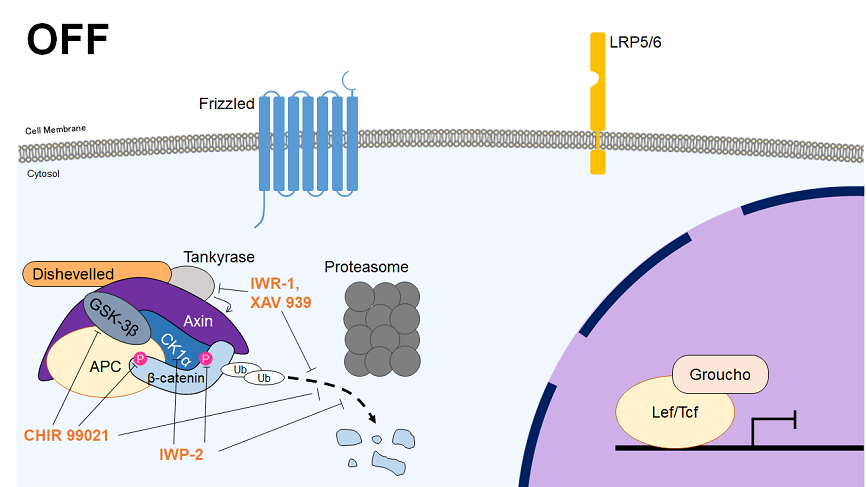
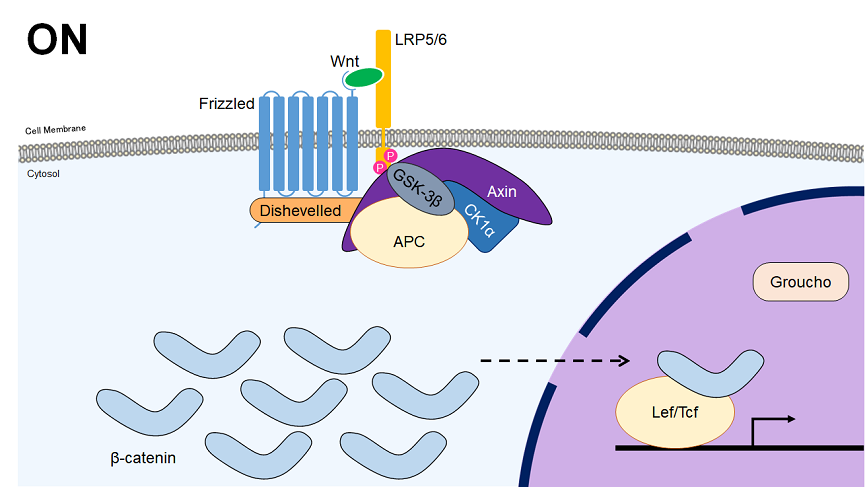
| Chemical Name | Target | Effect |
|---|---|---|
| CHIR 99021 | GSK-3α/β | Inhibitor |
| IWP-2 | CK1δ | Inhibitor |
| IWR-1 | Tankyrase | Inhibitor |
Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities
Cadherin Signaling Pathway
The cadherin family of genes play critical roles in calcium-dependent cell-cell contact and adhesion, in part mediating contact inhibition and epithelial-to-mesenchymal transition. The canonical cadherins are E-cadherin, N-cadherin, and P-cadherin, which associate with catenins to activate the Wnt, NFκB, Hippo, and RhoA signaling pathways.
| Chemical Name | Target | Effect |
|---|---|---|
| DAPT | γ-Secretase | Inhibitor |
Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Cadherin signaling: keeping cells in touch
- Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target
- Cleavage of E-Cadherin by Matrix Metalloproteinase-7 Promotes Cellular Proliferation in Nontransformed Cell Lines via Activation of RhoA
- ROCK Inhibition Facilitates the Generation of Human-Induced Pluripotent Stem Cells in a Defined, Feeder-, and Serum-Free System
Notch Signaling Pathway
The notch pathway is highly conserved among multicellular organisms due to its roles in cell-fate determination during early development as a mediator of cell/cell contact. Nascient notch receptor is transported to the cell membrane, where binding with such ligands as Jagged and DII cause it to be cleaved in turn by the ADAM family proteases and the γ-secretase complex. This results in liberation of notch’s intracellular domain (Notch-ICD), which is transported to the nucleus to act as a transcription factor upon complexing with CSL and MAML.

Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Notch signaling at a glance
- The Notch Ligands, Jagged and Delta, Are Sequentially Processed by α-Secretase and Presenilin/γ-Secretase and Release Signaling Fragments
Hedgehog Signaling Pathway
The Hedgehog (Hh) signaling pathway was first discovered due to the essential role it plays in body plan determination during development, but it has also been shown to be important for maintaining stem cell-ness in somatic stem cells. The mammalian Hedgehog ligands comprise three homologues (Desert – DHH, Indian – IHH, Sonic – SHH) with SHH being the most well studied. SHH binding to the Patched-1 (PTCH1) receptor abrogates PTCH1's repressive effect on Smoothened (SMO), which can then activate members of the GLI family of transcription factors, which go on to promote transcription of downstream factors.

Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Hedgehog Signaling in Development and Cancer
TGFβ Signaling Pathway
The receptors for the TGFβ signaling pathway are single-pass serine/threonine cell surface receptors, and are divided into two groups, type 1 and type 2 receptors. These receptors form a covalent disulfide bond with a receptor of the other type, forming a dimer which then itself dimerizes into a tetramer. The extracellular ligands this tetramer binds to, as well as the affinity and sensitivity of each subunit towards these ligands is used to separate the TGFβ family into three major groups, the TGFβ, BMP (Bone Morphogenic Protein), and Activin subgroups. Upon binding to a ligand, such proteins as Smad2/3, Smad1/5/8, and/or Tak1 are phosphorylated and activated, leading to an increase in the expression of genes related to embryonic development, cell proliferation and apoptosis. The TGFβ pathway is often dysregulated in cancer, and its inhibition is commonly required in organoid culture.

Products
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.
References
- Bone morphogenetic protein receptor signal transduction in human disease
- Opposing roles and potential antagonistic mechanism between TGF-β and BMP pathways: Implications for cancer progression
- Targeting transforming growth factor-β receptors in pulmonary hypertension
Other Organoid Culture Additives
Products
- A0905
- N-Acetyl-L-cysteine
- D1961
- Dexamethasone
- F0855
- Forskolin
- G0063
- L-Glutamine
- H0393
- Heparin Sodium Salt from Hog intestine
- M0058
- 2-Mercaptoethanol
- N0078
- Nicotinamide
- P1772
- Penicillin G Potassium Salt
- P1884
- Prostaglandin E2
- R0064
- Retinoic Acid
- S0585
- Streptomycin Sulfate
- T0027
- Testosterone
‡Our reagents are not explicitly guaranteed for cell culture. Please filter sterilize etc. before use.