The size of a fluorine atom is similar to a hydrogen atom but the electric property of it is more electronegative. So, by replacing a hydrogen atom of substances with a fluorine atom, their biological properties can be dramatically changed without a conformational change. Recently, the synthetic and biological studies of fluorinated molecules have attracted a lot of interest in the fields of pharmaceuticals and agrochemicals research. Especially, the synthetic study of the trifluoromethyl group-containing compounds is now actively proceeding.
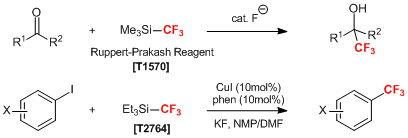
As a method for direct trifluoromethylation, nucleophilic and electrophilic/radical fluorinating reactions are commonly used. Ruppert-Prakash reagent (= (trifluoromethyl)trimethylsilane) (Product No. T0570) is the most popular nucleophilic trifluoromethylating reagent and which readily reacts with a fluoride ion to release the trifluoromethylanion species. This active species shows nucleophilicity and reacts with carbonyl compounds to proceed with the trifluoromethylation.1) Also, the nucleophilic trifluoromethylation of aromatic halides can be performed with the use of copper catalysts.2)
Published TCIMAIL newest issue No.200



![[(Oxido)phenyl(trifluoromethyl)-λ4-sulfanylidene]dimethylammonium Tetrafluoroborate [(Oxido)phenyl(trifluoromethyl)-λ4-sulfanylidene]dimethylammonium Tetrafluoroborate](/medias/O0367.jpg?context=bWFzdGVyfHJvb3R8MzA4MDd8aW1hZ2UvanBlZ3xhR000TDJnMVlpODRPVE14T0RNM056TTVNRE00TDA4d016WTNMbXB3Wnd8MDk4YmUxMmZjOTRiMmM0OWE3N2Q1NzMzNWM3YjRmMGJhMTcyNjdhMTlkZjEzM2QzZjc4ZDI2MjA3M2JiNWI3NA)









![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] (Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]](/medias/T1570.jpg?context=bWFzdGVyfHJvb3R8MzQ0NzB8aW1hZ2UvanBlZ3xhRGRsTDJnMk5pODRPVE15TkRnME16Z3lOelV3TDFReE5UY3dMbXB3Wnd8NjljMTU3YjNlZmU3NDg1ZTcwMDRkY2ViNDIyNTgwM2Q2NjlkYzEwYTUzZjQ1YzEyOGViYTFjMjliYWE4OGQyZQ)