Maximum quantity allowed is 999
请选择数量
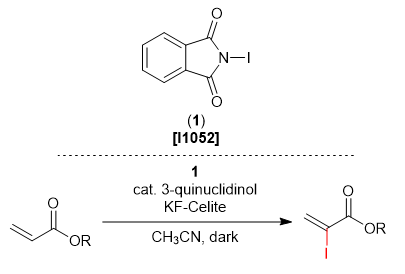
Phthalimide Derivative for Selective Iodination of Acrylic Esters
N-iodophthalimide (1) is utilized in the selective iodination at the α-position of acrylic esters.1) When acrylic esters react with 1 in the presence of a catalytic amount of 3-quinuclidinol and KF-Celite under light-shielded conditions, the iodination proceeds via a Morita-Baylis-Hillman type reaction to give α-iodoacrylic esters in good yields. This reaction proceeds only with acrylic esters and not with α,β-unsaturated esters bearing a substituent at the β-position. Given products can be applied in cross couplings such as the Nozaki-Hiyama-Kishi reaction. As per other reports, 1 has also been used as an iodine source in asymmetric iodination reactions.2)
Related Product
References
- 1) Highly efficient and chemoselective α-iodination of acrylate esters through Morita–Baylis–Hillman-type chemistry
- 2) Biomimetic Desymmetrization of a Carboxylic Acid