Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
CAS RN: 68373-14-8 | Product Number: S0868
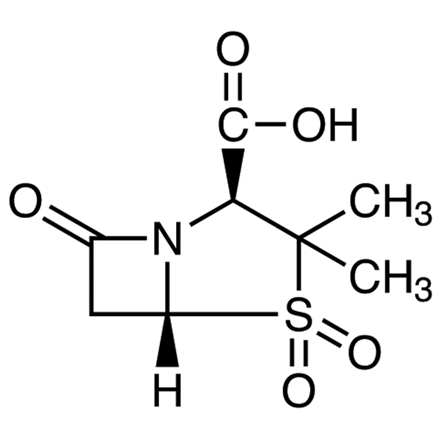
Sulbactam
Purity: >98.0%(T)(HPLC)
- (2S,5R)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid 4,4-Dioxide
| Size | Unit Price | Philadelphia, PA | Portland, OR | Japan* | Quantity |
|---|---|---|---|---|---|
| 5G |
$112.00
|
2 | 2 | 10 |
|
| 25G |
$378.00
|
1 | 2 | 2 |
|
* Items in stock locally ship in 1-2 business days. Items from Japan stock are able to ship from a US warehouse within 2 weeks. Please contact TCI for lead times on items not in stock. Excludes regulated items and items that ship on ice.
* To send your quote request for bulk quantities, please click on the "Request Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Product Number | S0868 |
| Purity / Analysis Method | >98.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__8H__1__1NO__5S = 233.24 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Frozen (<0°C) |
| Condition to Avoid | Heat Sensitive |
| CAS RN | 68373-14-8 |
| Reaxys Registry Number | 4192832 |
| PubChem Substance ID | 135727108 |
| Merck Index (14) | 8889 |
| MDL Number | MFCD00867005 |
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Neutralization titration) | min. 98.0 % |
| Specific rotation [a]20/D | +233.0 to +241.0 deg(C=1, H2O) |
| Melting Point | 156 °C(dec.) |
| Specific Rotation | 237° (C=1,H2O) |
| Solubility in water | Soluble |
| HS Number | 2941.10.5000 |
References
- Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important β-lactamases
- A Structure-Based Analysis of the Inhibition of Class A β-Lactamases by Sulbactam
- Inhibitor-resistant TEM β-lactamases: phenotypic, genetic and biochemical characteristics (a review)
- Understanding the longevity of the β-lactam antibiotics and of antibiotic/β-lactamase inhibitor combinations (a review)
- Current challenges in antimicrobial chemotherapy: focus on β-lactamase inhibition (a review)
Articles/Brochures
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.





