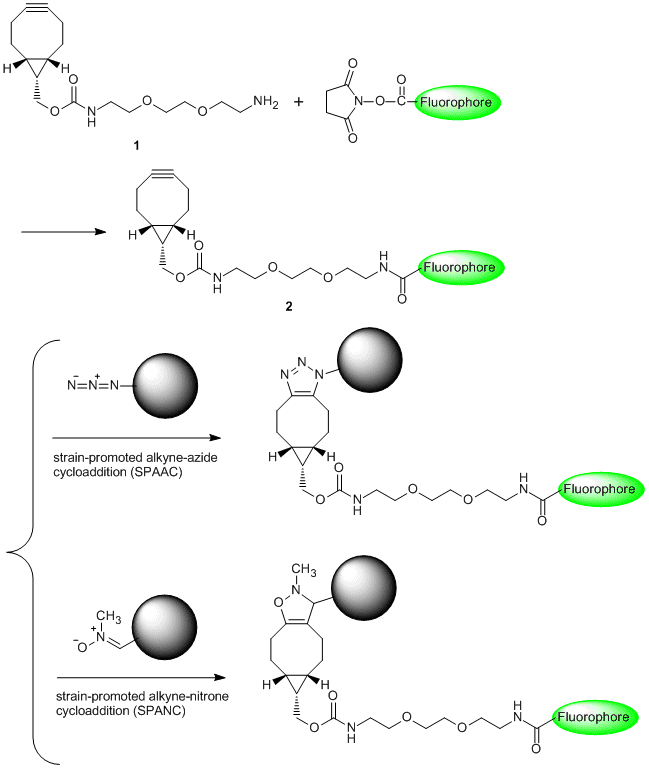
Huisgen [3+2] cycloaddition of azides with terminal alkynes is representative of "Click Chemistry" described by K. B. Sharpless in 2001. The reaction which affords products in high yield has been used for imaging labeling and tracking labeling of biomolecules. However, the reaction is not suitable for labeling of living systems because it needs a highly-concentrated copper(I) species.1)
BCN-amine (1) is a linker having a strained structure with cyclooctyne, and it is used for the copper-free click reaction to azides. For example, 1 bonded to a fluorophore (2) has resulted in labeling of an azidohomoalanine-containing virus capsid protein without copper(I) species.2) In addition, 1 can be applied to not only strain-promoted alkyne-azide cycloaddition (SPAAC)3) but also strain-promoted alkyne-nitrone cycloaddition (SPANC).4)
Maximum quantity allowed is 999
Please select the quantity
A Bioconjugatable Linker having a Copper-free "Click" Reaction to Azides
References
- Copper-free azide–alkyne cycloadditions: new insights and perspectives
- Readily accessible bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells
- A strain-promoted [3 + 2] azide–alkyne cycloaddition for covalent modification of biomolecules in living systems
- Protein modification by strain-promoted alkyne–nitrone cycloaddition