Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Please select the quantity
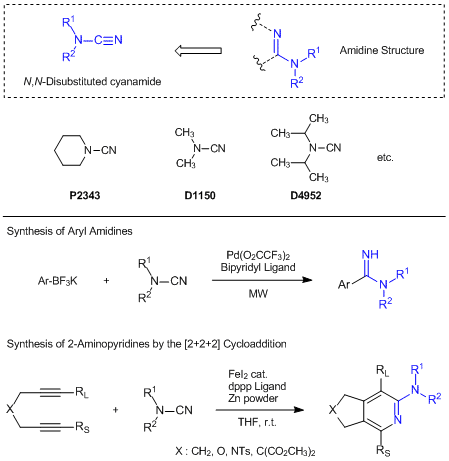
Useful N,N-Disubstituted Cyanamides for Introducing Amidine Moieties
N,N-Disubstituted cyanamides are composed from two functional groups of secondary amides and cyano groups, and which are effectively used for introducing amidine moieties. For instance, in the presence of bipyridyl ligands and palladium catalysts, piperidine-1-carbonitrile successfully reacts with aryltrifluoroborates under microwave conditions to afford the desired aryl amidine derivatives.1) On the other hand, 1,3-bis(diphenylphosphino)propane ligand and an iron catalysts/zinc powder catalytic-system can be applied to the [2+2+2] cycloaddition of N,N-disubstituted cyanamides with diynes and the favorable 2-aminopyridine derivatives are given.2) In this reaction, not only terminal diynes but also methyl group, phenyl group and TMS group substituted diynes are applicable to this cycloaddition and the 2-aminopyridine derivatives with substitutions of a higher bulky group at the 3-position are obtained preferably. In this way, N,N-disubstituted cyanamides can be used for the synthesis of aryl amidine derivatives and multi-substituted 2-aminopyridine derivatives.
Related Products
References
- 1)Direct Palladium(II)-Catalyzed Synthesis of Arylamidines from Aryltrifluoroborates
- 2)Iron-Catalyzed Cycloaddition Reaction of Diynes and Cyanamides at Room Temperature

