Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Please select the quantity
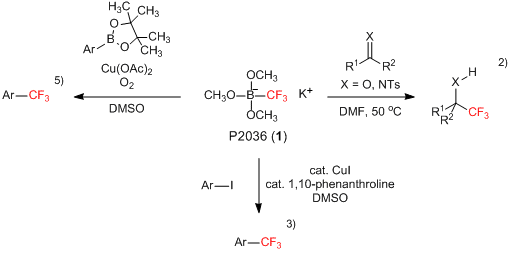
Organoboron Reagent Usable for Trifluoromethylating Reactions
Potassium trimethoxy(trifluoromethyl)borate (1) is a white solid which can be used as a trifluoromethylating reagent with easier handling.1-5) For example, 1 reacts with aldehydes, ketones and imines without α-protons under heating wherein the nucleophilic trifluoromethylation to their carbonyl or imino group proceeds in good yields.2) On the other hand, when substrates having α-protons are treated with 1, the trifluoromethylation competitively proceeds with the self-aldol condensation. Furthermore, by the use of copper salts with 1, cross coupling reactions with aryl iodides3) and aryl pinacolborane derivatives5) successfully proceed and the corresponding trifluoromethyl group-substituted aromatic compounds are given. In this way, 1 is an inexpensive and easier handling nucleophilic trifluoromethylating reagent and is expected to be used in organic synthesis.
Related Products
References
- 1)Perfluoroalkyl borates and boronic esters: new promising partners for Suzuki and Petasis reactions
- 2)Nucleophilic trifluoromethylation with organoboron reagents
- 3)Copper-Catalyzed Trifluoromethylation of Aryl Iodides with Potassium (Trifluoromethyl)trimethoxyborate
- 4)Catalyst-Controlled Regiodivergent C-H Borylation of Multifunctionalized Heteroarenes by Using Iridium Complexes
- 5)Oxidative Trifluoromethylation of Arylboronates with Shelf-Stable Potassium (Trifluoromethyl)trimethoxyborate

