Maximum quantity allowed is 999
CAS RN: 4525-65-9 | 產品號碼: T3121
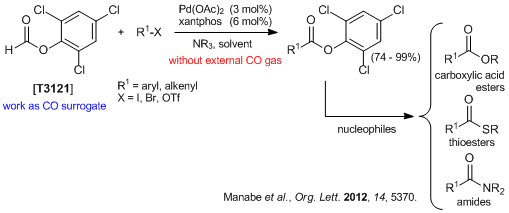
2,4,6-Trichlorophenyl Formate

* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | T3121 |
| 純度/分析方法 | >97.0%(HPLC) |
| 分子式 / 分子量 | C__7H__3Cl__3O__2 = 225.45 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 4525-65-9 |
| Reaxys-RN | 2524466 |
| PubChem Substance ID | 253661984 |
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 97.0 area% |
| Purity(Argentometric Titration) | min. 95.0 % |
| Melting point | 81.0 to 85.0 °C |
| 熔點 | 83 °C |
| 溶解性(可溶於) | Toluene |
| HS編碼* | 2915.13-000 |

-
Used Chemicals
-
Procedure
-
A solution of 2-iodofluorene (500 mg, 1.71 mmol), 2,4,6-trichlorophenyl formate (579 mg, 2.57 mmol), palladium(II) acetate (38.4 mg, 0.171 mmol), Xantphos (198 mg, 0.342 mmol) and triethylamine (470 µL, 3.42 mmol) in toluene (10 mL) was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure and the residue was washed with acetonitrile to give 1 as a pale yellow solid (462 mg, 69% yield).
A solution of 1 (460 mg, 1.18 mmol), triethylamine (330 µL, 2.36 mmol), DMAP (7.2 mg, 59 µmol) and morpholine (154 µL, 1.77 mmol) in THF (5 mL) was stirred at 45 °C for 20 hours. The reaction was quenched with water and the aqueous layer was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (ethyl acetate:hexane = 0:100 - 50:50) to give 2 as a white solid (305 mg, 93% yield).
-
Experimenter’s Comments
-
The reaction mixture at the first step was monitored by TLC (hexane:ethyl acetate = 1:19, Rf = 0.34).
The reaction mixture at the second step was monitored by TLC (hexane:ethyl acetate = 1:1, Rf = 0.26).
-
Analytical Data
-
2,4,6-Trichlorophenyl 9H-Fluorene-2-carboxylate (1)
(9H-Fluoren-2-yl)(morpholino)methanone (2)1H NMR (400 MHz, CDCl3);
(1) δ 8.41 (s, 1H), 8.29 (d, J = 8.1 Hz, 1H), 7.94-7.88 (m, 2H), 7.62 (d, J = 6.2 Hz, 1H), 7.48–7.38 (m, 4H), 4.02 (s, 2H).
(2) δ 7.81 (d, J = 7.6 Hz, 2H), 7.62-7.56 (m, 2H), 7.43-7.32 (m, 3H), 3.94 (s, 2H), 3.72 (brs, 8H). -
-
Lead Reference
-
- Trichlorophenyl Formate: Highly Reactive and Easily Accessible Crystalline CO Surrogate for Palladium-Catalyzed Carbonylation of Aryl/Alkenyl Halides and Triflates.
-
Other References
-
- Pd-Catalyzed External-CO-Free Carbonylation: Preparation of 2,4,6-Trichlorophenyl 3,4-Dihydronaphthalene-2-Carboxylate
- Formic Acid Derivatives as Practical Carbon Monoxide Surrogates for Metal-Catalyzed Carbonylation Reactions
References
- 1)T. Ueda, H. Konishi, K. Manabe, Org. Lett. 2012, 14, 5370.

- 2)H. Konishi, T. Ueda, K. Manabe, Org. Synth. 2014, 91, 39.

- 3)H. Konishi, K. Manabe, Synlett 2014, 25, 1971.





