Maximum quantity allowed is 999
CAS RN: 358-23-6 | 產品號碼: T1100
Trifluoromethanesulfonic Anhydride
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| Appearance | Colorless to Almost colorless clear liquid |
| Purity(Neutralization titration) | min. 98.0 % |
| NMR | confirm to structure |
| 熔點 | -80 °C |
| 沸點 | 84 °C |
| 比重 | 1.72 |
| 折射率 | 1.32 |
| 圖形表示 |

|
| 信號詞 | Danger |
| 危險性說明 | H314 : Causes severe skin burns and eye damage. H290 : May be corrosive to metals. |
| 防範說明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P234 : Keep only in original container. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P390 : Absorb spillage to prevent material damage. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P406 : Store in corrosive resistant container with a resistant inner liner. P405 : Store locked up. |
| UN編號 | UN3265 |
| 類別 | 8 |
| 包裝類別 | II |
| HS編碼* | 2904.10-000 |

-
Used Chemicals
-
Procedure
-
Under nitrogen atmosphere, triphenylphosphine oxide (1.057 g, 3.798 mmol) was dissolved in dichloromethane (20 mL) and cooled to 0 °C. Then trifluoromethanesulfonic anhydride anhydride (0.295 mL, 1.80 mmol) was added and stirred for 1 h. After confirming the formation of intermediate 1 by 31P-NMR, PPTS (0.452 g, 1.80 mmol) was added and the mixture was stirred for 30 min. After that, a solution of triethylamine (0.28 mL, 2.00 mmol) and pentafluorophenol (0.370 g, 2.00 mmol) in dichloromethane (5 mL) was added dropwise with a syringe to this solution. Then the mixture was warmed to room temperature and stirred for 1 h. After completion of the reaction, the mixture was diluted with dichloromethane (20 mL) and then 2 mol/L HCl (30 mL) was added to quench. The two layers were separated and the organic layer was washed with brine (20 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel chromatography (hexane:ethyl acetate = 9:1) to afford pentafluorophenyl p-toluenesulfonate as a yellow solid (540 mg, 88% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by 1H-NMR and 31P-NMR (CDCl3).
Dichloromethane was used for the reaction after dehydration with molecular sieves and bubbling with nitrogen.
-
Analytical Data
-
Pentafluorophenyl p-Toluenesulfonate
1H NMR (400 MHz, CDCl3); δ 7.86 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.2 Hz, 2H), 2.51(s, 3H).
-
Lead Reference
-
- Direct Synthesis of Sulfonamides and Activated Sulfonate esters From Sulfonic Acids
-
Other Reference
-
- The Structure of Polymer-Supported Triphenylphosphine Ditriflate: A Potentially Useful Reagent in Organic Synthesis

-
Used Chemicals
-
Procedure
-
Tf2O (0.32 mL, 2.0 mmol, 1.3 eq.) was slowly added to a solution of 4-bromobenzamide (300 mg, 1.5 mmol), and triethylamine (0.54 mL, 3.9 mmol, 2.6 eq.) in dichloromethane (3 mL) at 0 °C. The mixture was stirred at room temperature for 2 hours. Then additional triethylamine (1.1 mL, 7.8 mmol, 5.2 eq.) and Tf2O (0.64 mL, 3.9 mmol, 2.6 eq.) were added at 0 °C and the mixture was stirred at r.t. for overnight. The reaction mixture was quenched with water (15 mL) and the aqueous layer was extracted with ethyl acetate (15 mL x 3). The combined organic layer was washed with 2 mol/L HCl aq. (15 mL), sat. NaHCO3 aq. (15 mL), and brine (15 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (on silica gel, ethyl acetate:hexane = 1:8) to give 4-bromobenzonitrile a pale yellow solid (165 mg, 61% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by UPLC.
-
Analytical Data
-
4-bromobenzonitrile
1H NMR (400 MHz, CDCl3); δ 7.64 (d, J = 8.4 Hz, 2H), 7.53 (d, J = 8.4 Hz, 2H).
-
Lead Reference
-
- A Practical Method for the Preparation of Nitriles from Primary Amides Under Non-Acidic Conditions
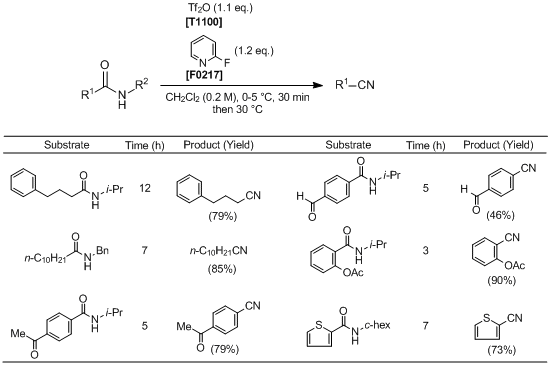
To an ice bath-cooled solution of a secondary amide (1.0 mmol) and 2-fluoropyridine (1.2 mmol) in anhydrous CH2Cl2 (5 mL) is added Tf2O (1.1 mmol). After being stirred for 0.5 h, the reaction mixture is warmed to 30 °C and stirred until completion of the reaction (monitored by TLC analysis). The reaction is quenched with 1 M HCl (1.0 mL), and the mixture is extracted with CH2Cl2 (3×10 mL). The combined organic layers are washed with saturated sodium carbonate (5 mL) and brine (5 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue is purified by column chromatography on silica gel (ethyl acetate/petroleum ether) to afford the corresponding nitrile.
References
References
- J. E. McMurry, W. J. Scott, Tetrahedron Lett. 1980, 21, 4313.

- H. J. Bestmann, K. Kumar, W. Schaper, Angew. Chem. Int. Ed. Engl. 1983, 22, 167.

- P. J. Stang, M. Hanack, L. R. Subramanian, Synthesis 1982, 85.

文章/手冊
[TCI Practical Example] Dehydration of a Carbamoyl Group Using Trifluoromethanesulfonic Anhydride
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜
很抱歉,您搜索的分析圖譜無法提供。




