Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
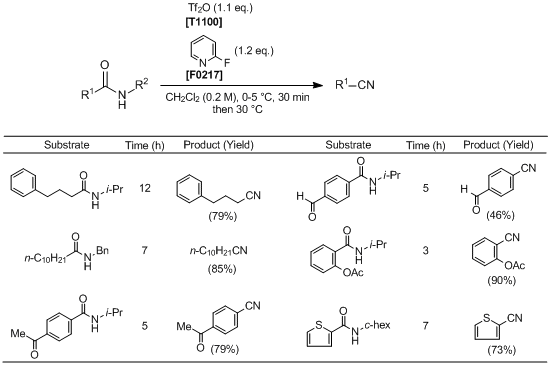
Transformation of Aliphatic and Aromatic Secondary Amides to Nitriles
Huang et al. have reported the transformation of aliphatic and aromatic secondary amides to nitriles. According to their results, by using trifluoromethanesulfonic anhydride in combination with 2-fluoropyridine, secondary amides are successfully transformed into the corresponding nitriles with the elimination of an N-alkyl group under mild reaction conditions. This reaction effectively proceeds when using amides bearing either secondary, tertiary, or benzylic N-alkyl groups. In addition, the reaction also tolerates several labile functional groups such as esters, ketones and aldehydes. Thus this synthetic approach has attracted much attention as a useful synthetic method for nitriles.