Maximum quantity allowed is 999
Please select the quantity
CAS RN: 911482-75-2 | Product Number: A2574
2-Allyl-5,5-dimethyl-1,3,2-dioxaborinane (stabilized with Phenothiazine)

Purity: >98.0%(GC)(T)
Synonyms:
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 1G |
$114.00
|
1 | 0 | Contact Us | |
| 5G |
$369.00
|
4 | 19 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | A2574 |
| Purity / Analysis Method | >98.0%(GC)(T) |
| Molecular Formula / Molecular Weight | C__8H__1__5BO__2 = 154.02 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive,Heat Sensitive |
| CAS RN | 911482-75-2 |
| Reaxys Registry Number | 11063535 |
| PubChem Substance ID | 253660089 |
Specifications
| Appearance | Colorless to Light yellow to Light orange clear liquid |
| Purity(GC) | min. 98.0 % |
| Purity(Neutralization titration) | min. 98.0 % |
Properties (reference)
| Flash point | 56 °C |
| Specific Gravity (20/20) | 0.93 |
| Refractive Index | 1.44 |
GHS
| Pictogram |


|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. H412 : Harmful to aquatic life with long lasting effects. H226 : Flammable liquid and vapor. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P273 : Avoid release to the environment. P240 : Ground/bond container and receiving equipment. P210 : Keep away from heat/sparks/open flames/hot surfaces. No smoking. P233 : Keep container tightly closed. P243 : Take precautionary measures against static discharge. P241 : Use explosion-proof electrical/ ventilating/ lighting/ equipment. P242 : Use only non-sparking tools. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P403 + P235 : Store in a well-ventilated place. Keep cool. |
Related Laws:
Transport Information:
| UN Number | UN1993 |
| Class | 3 |
| Packing Group | III |
| H.S.code* | 2934.99-000 |
Application
Catalytically High Efficiency Allylating Reaction
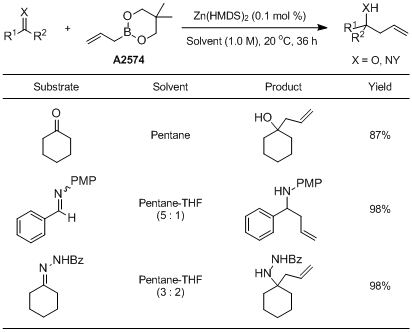
Example: Catalytic allylation of cyclohexanone
To a dried septum-capped 10 mL-flask with magnetic stirring bar under an argon atmosphere is added Zn(HMDS)2 (1.2 mg, 0.0030 mmol, 0.10 mol%). After addition of dry pentane (3.0 mL, 1.0 M), allylboronate (508 mg, 560µL, 3.30 mmol) and cyclohexanone (294 mg, 3.00 mmol) are added. The mixture is stirred under an argon atmosphere at 20 °C for 36 h. After dilution with ethyl acetate is added a saturated aqueous NH4Cl and the phases are separated. The aqueous phase is then extracted with ethyl acetate (15 mL x 3) and the combined organic layers are dried on Na2SO4, filtered and concentrated in vacuo. The residue is purified by silica gel column chromatography (eluent: n-hexane/ethyl acetate = 15:1 to 2:1) to afford the corresponding homoallylic alcohol in 89% yield.
To a dried septum-capped 10 mL-flask with magnetic stirring bar under an argon atmosphere is added Zn(HMDS)2 (1.2 mg, 0.0030 mmol, 0.10 mol%). After addition of dry pentane (3.0 mL, 1.0 M), allylboronate (508 mg, 560µL, 3.30 mmol) and cyclohexanone (294 mg, 3.00 mmol) are added. The mixture is stirred under an argon atmosphere at 20 °C for 36 h. After dilution with ethyl acetate is added a saturated aqueous NH4Cl and the phases are separated. The aqueous phase is then extracted with ethyl acetate (15 mL x 3) and the combined organic layers are dried on Na2SO4, filtered and concentrated in vacuo. The residue is purified by silica gel column chromatography (eluent: n-hexane/ethyl acetate = 15:1 to 2:1) to afford the corresponding homoallylic alcohol in 89% yield.
References
- Catalytic Use of Zinc Amide for Transmetalation with Allylboronates: General and Efficient Catalytic Allylation of Carbonyl Compounds, Imines, and Hydrazones
- Facile preparation of allylzinc species from allylboronates and zinc amide via a boron-to-zinc exchange process and their reactions with carbonyl compounds, imines and hydrazones
PubMed Literature
Articles/Brochures
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



