Maximum quantity allowed is 999
请选择数量
产品 > TCI热门产品信息 > TCI热门产品信息 2016 >
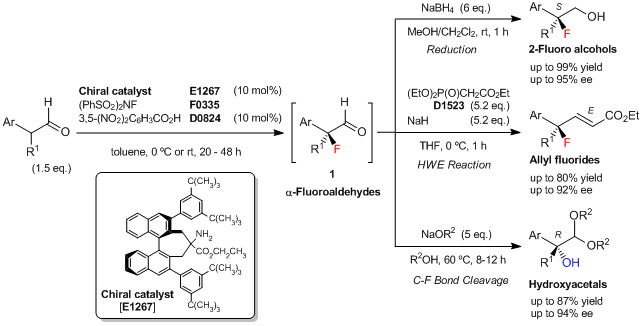
使用最新开发的手性胺催化剂用于α-支链醛的对映选择性氟化反应
Shibatomi等人报道了一种新型的α-支链醛的对映选择性氟化反应,该反应使用了最新开发的手性联萘伯胺催化剂。在10 mol%手性胺催化剂和10 mol% 3,5-二硝基苯甲酸的共同催化作用下,α-支链醛与N-氟代苯磺酰亚胺(NFSI)进行了不对称氟化反应,并生成了相应的α-氟代醛(1)。1难以纯化,不过可以转化为各种手性叔氟化物。比如,还原后可得到2-氟代伯醇,通过Horner–Wadsworth–Emmons (HWE)反应可得到烯丙基氟化物。另外,通过立体专一性的C-F键裂解,1还可以转化为相应的含有叔醇结构的手性α-羟基缩醛。