Maximum quantity allowed is 999
CAS RN: 133745-75-2 | 产品编码: F0335
N-Fluorobenzenesulfonimide [Fluorinating Reagent]
![N-Fluorobenzenesulfonimide [Fluorinating Reagent] No-Image](/medias/F0335.jpg?context=bWFzdGVyfHJvb3R8NDYzMDV8aW1hZ2UvanBlZ3xhREZrTDJnMFlTODRPVEl4TmpnNU16VTRNelkyTDBZd016TTFMbXB3Wnd8NDJkMWVkY2MxYjM2N2FjOWNlNDk2ZjkxOWJhNjljYWEwNmQzMzY5ZTBhYWFlMDYyOGMxM2M5NmIzNjA3MmVhOQ)
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Iodometric Titration) | min. 98.0 % |
| Melting point | 115.0 to 119.0 °C |
| 熔点 | 115 °C |
| 象形图 |


|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. H341 : Suspected of causing genetic defects. |
| 防范说明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P202 : Do not handle until all safety precautions have been read and understood. P201 : Obtain special instructions before use. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P308 + P313 : IF exposed or concerned: Get medical advice/ attention. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P405 : Store locked up. |
| HS编码* | 2935.90-000 |

-
Used Chemicals
-
Procedure
-
To a solution of 4-bromobiphenyl (1.17 g, 5.00 mmol), DABSO (721 mg, 3.00 mmol), and PdCl2(Amphos)2 (177 mg, 0.25 mmol) in anhydrous isopropyl alcohol (20 mL) was added triethylamine (2.1 mL, 15 mmol) at room temperature and the mixture was stirred at 75 °C for 23 hours. The suspension was cooled at room temperature and NFSI (2.36 g, 7.5 mmol) was added and the reaction mixture stirred for 3 hours until completion. The solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (20 mL) and filtrated through celite pad. The filtrate was washed with saturated aqueous Na2S2O3 solution (20 mL) and brine (20 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 2:98 - 5:95 on silica gel) to give compound 1 as a white solid (928 mg, 79%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.57) and UPLC-MS.
-
Analytical Data
-
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.12–8.04 (m, 2H), 7.86–7.80 (m, 2H), 7.67–7.58 (m, 2H), 7.44-7.55 (m, 3H).
-
Lead Reference
-
- One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides
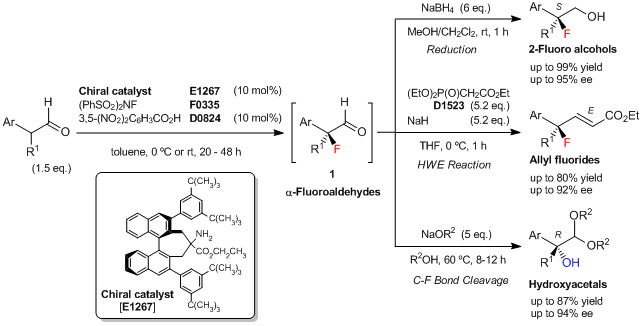
To a solution of the chiral amine catalyst [E1267] (20 mg, 0.026 mmol, 10 mol%) in toluene (0.54 mL) is added 3,5-dinitrobenzoic acid (5.5 mg, 0.026 mmol, 10 mol%), 2-phenylpropionaldehyde (52 mg, 0.39 mmol, 1.5 eq.), and N-fluorobenzenesulfonimide (NFSI) (0.26 mmol, 82 mg, 1 eq.) at 0 °C. The reaction mixture is stirred at 0 °C for 48 h, then poured into CH3OH/CH2Cl2 (1:4, 1.3 mL) at 0 °C. To this solution, NaBH4 (1.6 mmol, 6 eq.) is added, and the mixture is stirred at room temperature for 1 h. The reaction is quenched with saturated aq.NH4Cl, and the mixture is extracted with Et2O. The organic layer is dried over Na2SO4, and concentrated. The residue is purified by silica gel chromatography (eluent: hexane/ethyl acetate = 3/1) to give (S)-2-fluoro-2-phenylpropan-1-ol (34.5 mg, 86% yield based on NFSI, 95% ee) as a white solid.
References

In a dry 25 mL round-bottom flask, thiophene (0.5 mmol, 1 eq.), NFSI (0.6 mmol, 1.2 eq.) and CuI (5 mol%) are dissolved in DCE (3 mL), the resulting mixture is put into a preheated oil bath (60 °C) for 8 h. The solution is then cooled to room temperature, evaporated under reduced pressure, diluted with ethyl acetate, washed with a saturated aqueous solution of NaCl, dried over Na2SO4, filtered and evaporated under reduced pressure. The crude product is purified by silica gel column chromatography to afford the corresponding product (166.7 mg, Y. 88 %).
References

Reference
文章/手册
[研究论文] 铜催化杂环化合物和N-氟代苯磺酰亚胺的直接酰胺化反应
[TCI应用实例] 使用DABSO和NFSI对芳基溴化物进行一锅法钯催化的氟磺酰化反应
化学品安全说明书(SDS)
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
示例 CoA
目前没有该产品的 CoA 示例。
分析图谱
很抱歉,您搜索的分析图谱无法提供。



![N-Fluorobenzenesulfonimide [Fluorinating Reagent] N-Fluorobenzenesulfonimide [Fluorinating Reagent]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)


