Maintenance Notice (10:30 PM January 4 - 8:00 AM January 5, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197 | Notice of Discontinuing the Use of Password-Protected Compressed Files | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
CAS RN: 1232692-92-0 | 产品编码: D4264
2-(Diisopropylsilyl)pyridine

技术规格
| Appearance | Colorless to Light orange to Yellow clear liquid |
| Purity(GC) | min. 95.0 % |
| NMR | confirm to structure |
物性(参考值)
| 闪点 | 84 °C |
| 比重 | 0.90 |
| 折射率 | 1.49 |
GHS
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. H227 : Combustible liquid. |
| 防范说明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P210 : Keep away from heat/sparks/open flames/hot surfaces. No smoking. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P403 + P235 : Store in a well-ventilated place. Keep cool. |
相关法规
运输信息
| HS编码* | 2933.39-000 |
应用
Introduction of the Pyridyldiisopropylsilyl (PyDipSi) Group into Aromatic Rings

To a solution of aryl bromide or -iodide (10 mmol) in THF (15 mL) is added dropwise a solution of n-butyllithium (3.86 mL, 2.59 M in hexanes, 10 mmol) at -78 °C under argon atmosphere. The reaction mixture is stirred at -78 °C for 15 – 40 min and then neat 2-(diisopropylsilyl)pyridine (1.934 g, 10.0 mmol) is added dropwise. The resulting solution is stirred at -78 °C for 1 h and then is allowed to warm to -30 °C. Upon completion of the reaction, the mixture is poured into 150 mL of hexanes containing small amount of water to neutralize LiH. The resulting solution is dried over anhydrous sodium sulfate, filtered, and concentrated under a reduced pressure. The residue is purified by flash column chromatography (silica gel, eluent: EtOAc/Hexanes = 1/10 –1/2) to provide aryl pyridyl silane.
References
- A. S. Dudnik, N. Chernyak, C. Huang, V. Gevorgyan, Angew. Chem. Int. Ed. 2010, 49, 8729.

- N. Chernyak, A. S. Dudnik, C. Huang, V. Gevorgyan, J. Am. Chem. Soc. 2010, 132, 8270.

应用
Halogenation of Aryl Pyridyl Silanes
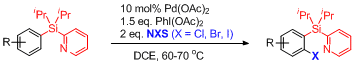
An oven dried 10 ml Wheaton V-vial, containing a stirring bar, is charged with 2-(diisopropyl(aryl)silyl)pyridine (0.5 mmol), Pd(OAc)2 (11.2 mg, 0.05 mmol), PhI(OAc)2 (241.6 mg, 0.75 mmol), and NXS (1.0 mmol) under N2 atmosphere. Dry DCE (5 mL) is added and the reaction vessel is capped with pressure screw cap. Reaction mixture is heated at 60 – 70 °C for 45 min – 4 h until judged complete by GC/MS analysis. The resulting mixture is cooled down to room temperature and filtered through a layer of silica gel with the aid of EtOAc. The filtrate is concentrated under a reduced pressure and the residue is purified by column chromatography on a silica gel (eluent: hexanes/EtOAc = 1/10 – 1/2) affording the corresponding halogenated product.
References
参考文献
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)






