Maximum quantity allowed is 999
请选择数量
CAS RN: 12112-67-3 | 产品编码: C1807
Chloro(1,5-cyclooctadiene)iridium(I) Dimer

| 产品编码 | C1807 |
| 纯度/分析方法 | >93.0%(T) |
| 分子式/分子量 | C__1__6H__2__4Cl__2Ir__2 = 671.70 |
| 外观与形状(20°C) | 固体 |
| 储存温度 | 冷藏 (0-10°C) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 空气,加热 |
| 包装和容器 | 1G-Glass Bottle with Plastic Insert (查看图片), 250MG-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 12112-67-3 |
| Reaxys-RN | 14372408 |
| PubChem物质ID | 87560730 |
| MDL编号 | MFCD00012414 |
技术规格
| Appearance | Orange to Amber to Dark red powder to crystal |
| Purity(Argentometric Titration) | min. 93.0 % |
物性(参考值)
| 熔点 | 205 °C(dec.) |
| 水溶性 | 不溶 |
GHS
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相关法规
运输信息
| HS编码* | 2843.90-000 |
应用
Iridium / Axially Chiral Phosphoramidite Complex Catalyzed Allylic Substitution of Allyl Carbonate Derivatives

In a nitrogen-filled atmosphere, [Ir(cod)Cl]2 (2.7 mg, 0.0040 mmol), the ligand (4.3 mg, 0.0080 mmol), KF (12 mg, 0.20 mmol), 18-crown-6 (53 mg, 0.20 mmol) and a Teflon-coated magnetic stirring bar are added to a 1-dram vial. Then, anhydrous THF (0.40 mL) is added. The mixture is stirred for 5 min. at ambient temperature before allyl carbonate derivative (0.20 mmol) and the silyl enolate derivative (0.40 mmol, 2.0 equiv) are added. The vial is sealed with a cap and stirred at 50 °C for 12 h. Then, water (1 mL) is added to the reaction mixture followed by 1 N HCl (0.5 mL). The resulting mixture is stirred vigorously for 3 h at room temperature. Brine (1 mL) and Et2O (1 mL) are added; the organic layer is separated, and the aqueous layer is extracted with Et2O (3 x 3 mL). The combined organic extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product is performed by flash column chromatography (gradient elution: hexane:Et2O = 50:1 to 5:1) to provide the product.
References
- Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones
应用
C-H Borylation of Arenes

Typical Procedure:
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
References
- Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate
应用
Iridium-catalyzed reaction of alcohols with enones affording 1,3-diketones
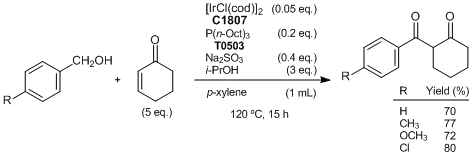
Typical procedure (R=H):
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
References
应用
Iridium-catalyzed alpha-alkylation of tert-butyl acetate

Typical procedure:
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
References
应用
Selective Synthesis of Diketones and omega-Hydroxy Ketones by [IrCl(cod)]2/PPh3/KOH System
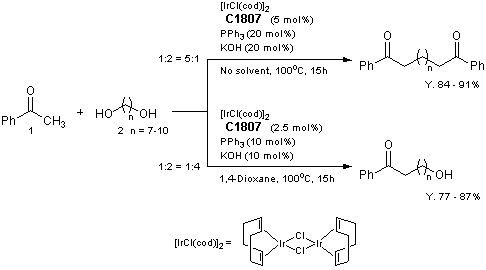
Reference
参考文献
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






