Maximum quantity allowed is 999
Please select the quantity
CAS RN: 929896-28-6 | Product Number: B4195
Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium
Purity:
Synonyms:
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 10MG |
$242.00
|
0 | 0 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | B4195 |
| Molecular Formula / Molecular Weight | C__2__4H__3__5N__2O__7Rh = 566.46 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 929896-28-6 |
| PubChem Substance ID | 253659893 |
Specifications
| Appearance | Light yellow to Yellow to Orange powder to crystal |
| Elemental analysis(Nitrogen) | 4.40 to 5.20 % |
| NMR | confirm to structure |
Properties (reference)
GHS
Related Laws:
Transport Information:
| H.S.code* | 2843.90-000 |
Application
Pincer Type Rh Complex Catalyzed Asymmetric Diboration of Terminal Alkenes and Their Conversion to the Optically Active 1,2-Diols
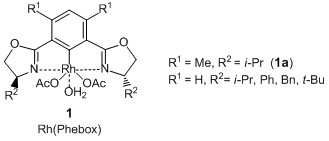

Nishiyama et al. have reported that optically active 1,2-diols are given by the pincer type Rh complexes [Rh(Phebox), 1] catalyzed asymmetric 1,2-diboration of terminal alkenes and subsequent oxidation. According to their results, the asymmetric diboration of 4-chlorostyrene with a diboron ester proceeds with high enantioselectivity by using a rhodium complex coordinated with a pincer type ligand (1a), and the desired 1,2-diborylated product is formed. Subsequent oxidation of it with sodium peroxoborate in THF-water gives the corresponding 1,2-diol. In this reaction, allyl ethers and allyl anilines can be transformed into the related optically active 1,2-diols. In addition, when using trans 1,3-dienes as reactants, terminal alkene moieties are selectively reacted and the optically active allylic alcohols are given. Thus, this asymmetric diboration using 1 is an efficient synthetic method to produce the optically active 1,2-diols.
References
- Asymmetric diboration of terminal alkenes with a rhodium catalyst and subsequent oxidation: enantioselective synthesis of optically active 1,2-diols
PubMed Literature
Product Articles
[TCIMAIL No.160] Pincer Type Rh Complex Catalyzed Asymmetric Diboration of Terminal Alkenes and Their Conversion to the Optically Active 1,2-DiolsProduct Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.
![Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium Thumbnail of Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium](/medias/B4195.jpg?context=bWFzdGVyfHJvb3R8MTc3NDB8aW1hZ2UvanBlZ3xhR0UxTDJobU1pODRPVE16TmpjeU1Ea3hOamM0TDBJME1UazFMbXB3Wnd8YjUzYTM1YjZlZGNhYmU3OGMxM2EyOTkyNWQyYWZjNzc3NDBhMDEwZGZkZmFmOTNhOTVjOWY0NjQzY2YzMmY5MA)
![Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium Chemical Structure of Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium](/medias/B4195.jpg?context=bWFzdGVyfHJvb3R8NjUyNzd8aW1hZ2UvanBlZ3xhRGsxTDJnMFlpODRPVEl3TWpVNE1UZ3pNVGs0TDBJME1UazFMbXB3Wnd8NTM3ZDVkNGU3NWI1NjY5Y2VhNjM2YzAzODdmM2U3MjhhOGVhNjM1ZGM0MTExNGM2MWJmNjgyYjE5NWFjM2YyNQ)
![Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium Bis(acetato)aqua[(S,S)-4,6-bis(4-isopropyl-2-oxazolin-2-yl)-m-xylene]rhodium](/_ui/responsive/theme-tci/images/missing_product_zoom.webp)

