Maximum quantity allowed is 999
Please select the quantity
CAS RN: 311-28-4 | Product Number: T0057
Tetrabutylammonium Iodide

Purity: >98.0%(T)
Synonyms:
- TBAI
Product Documents:
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | T0057 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__1__6H__3__6IN = 369.38 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Light Sensitive,Hygroscopic |
| CAS RN | 311-28-4 |
| Reaxys Registry Number | 3916152 |
| PubChem Substance ID | 87576076 |
| SDBS (AIST Spectral DB) | 7778 |
| MDL Number | MFCD00011636 |
Specifications
| Appearance | White to Almost white powder to crystaline |
| Purity(Nonaqueous Titration) | min. 98.0 % |
| Melting point | 144.0 to 149.0 °C |
| Solubility in hot Water | almost transparency |
Properties (reference)
| Melting Point | 147 °C |
| Solubility (soluble in) | Alcohol, Ether |
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H302 : Harmful if swallowed. H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. |
Related Laws:
| RTECS# | BS5450000 |
Transport Information:
| H.S.code* | 2923.90-000 |
Application
Deprotection of Bn Groups using Phenyl Trimethylsilyl Sulfide

Reference
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Cleavage of Methoxy groups using Aluminum(III) Chloride and TBAI


Reference
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Direct α-Oxyacylation of Carbonyl Compounds
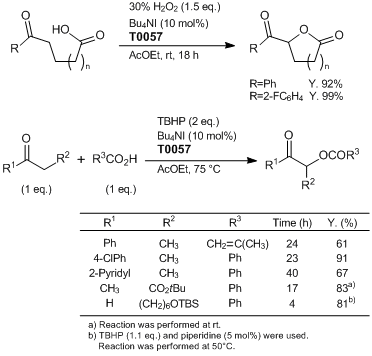
Typical Procedure: α-Oxyacylation of Ketones
To a stirring mixture of ketone (1 mmol), carboxylic acid (1 mmol) and Bu4NI (36.9 mg, 0.1 mmol, 10 mol%) in AcOEt (5 mL) is added tert-butyl hydroperoxide (5.5 M in nonane or decane, 364 µL, 2 mmol) at room temperature. The resulting mixture is heated to 75 °C. The reaction is monitored by TLC analysis. After the reaction is completed, the reaction mixture is cooled to room temperature and poured into sat. Na2S2O3 aq. (5 mL) and sat. NaHCO3 aq. (5 mL) extracted with AcOEt (twice), and washed with brine. The combined organic layers are dried over anhydrous Na2SO4 and solvents are removed in vacuo. The residue is purified by flash column chromatography on silica gel (hexane–AcOEt as eluent) to give the corresponding analytically pure product.
To a stirring mixture of ketone (1 mmol), carboxylic acid (1 mmol) and Bu4NI (36.9 mg, 0.1 mmol, 10 mol%) in AcOEt (5 mL) is added tert-butyl hydroperoxide (5.5 M in nonane or decane, 364 µL, 2 mmol) at room temperature. The resulting mixture is heated to 75 °C. The reaction is monitored by TLC analysis. After the reaction is completed, the reaction mixture is cooled to room temperature and poured into sat. Na2S2O3 aq. (5 mL) and sat. NaHCO3 aq. (5 mL) extracted with AcOEt (twice), and washed with brine. The combined organic layers are dried over anhydrous Na2SO4 and solvents are removed in vacuo. The residue is purified by flash column chromatography on silica gel (hexane–AcOEt as eluent) to give the corresponding analytically pure product.
References
PubMed Literature
Articles/Brochures
TCIMAIL
[Product Highlights] Trifluoromethylthiolating Agent Usable for Direct Dehydroxytrifluoromethylthiolation of Alcohols[Research Articles] Direct α-Oxyacylation of Carbonyl Compounds
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




