Maintenance Notice (3:15 - 7:30 AM February 22, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Notice of Discontinuing the Use of Password-Protected Compressed Files | Product Document Searching Made Easy by 2D Code! | TCI Life Science News February 2025 | [Product Highlights] Chemiluminescent Reagents for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
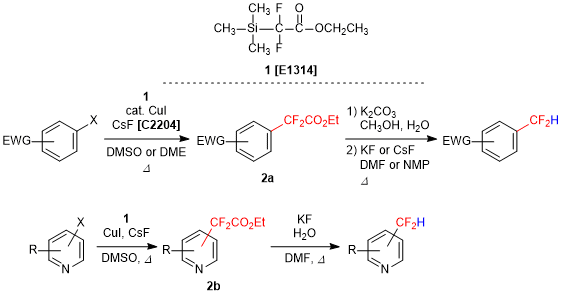
Difluoroacetate Derivative for the Synthesis of Difluoromethylaryls
Ethyl 2,2-difluoro-2-(trimethylsilyl)acetate (1) is utilized to introduce a difluoromethyl group in aromatic ring compounds. In the presence of copper(I) iodide and cesium fluoride, (hetero)aryl halides react with 1 to give 2-aryldifluoromethylacetate esters 2a and 2b. Both 2a and 2b can be subsequently converted into difluoromethyl aryl compounds via hydrolysis and decarboxylation.1) Furthermore, pyridine derivative 2b affords corresponding difluoromethyl compounds in a single operation upon treatment with potassium fluoride in water.2) Difluoromethyl group has a high lipophilicity and an electro-withdrawing power, and also behaves as a hydrogen bond donor, so it is focused on the researches of pharmaceuticals and agrochemicals. As a result, 1 is anticipated as a promising compound for the introduction of difluoromethyl group.
Related Products
References
- 1) A New Method for Aromatic Difluoromethylation: Copper-Catalyzed Cross-Coupling and Decarboxylation Sequence from Aryl Iodides
- 2) An Efficient Route to Difluoromethylated Pyridines