Maximum quantity allowed is 999
Please select the quantity
CAS RN: 12112-67-3 | 제품번호: C1807
Chloro(1,5-cyclooctadiene)iridium(I) Dimer

•본 제품의 재고는 일본 본사의 재고입니다. 주문시, 5일안에 실험실까지 도착됩니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | C1807 |
| Purity/Analysis Method | >93.0%(T) |
| M.F. / M.W. | C__1__6H__2__4Cl__2Ir__2 = 671.70 |
| 물리적 상태 (20 ℃) | Solid |
| 보관 조건 | Refrigerated (0-10°C) |
| 불활성 가스 하에서 보관 | Store under inert gas |
| 피해야 할 조건 | Air Sensitive,Heat Sensitive |
| 용기 | 1G-Glass Bottle with Plastic Insert (이미지 보기), 250MG-Glass Bottle with Plastic Insert (이미지 보기) |
| CAS RN | 12112-67-3 |
| Reaxys-RN | 14372408 |
| PubChem Substance ID | 87560730 |
| MDL 번호 | MFCD00012414 |
규격표
| Appearance | Orange to Amber to Dark red powder to crystal |
| Purity(Argentometric Titration) | min. 93.0 % |
물성치(참고치)
| mp | 205 °C(dec.) |
| Solubility in water | Insoluble |
GHS
| 픽토그램 |

|
| 신호 워드 | Warning |
| 위험물 및 유해 등록 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 주의 사항 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
법규 정보
운송 정보
| HS 번호* | 2843.90-000 |
Application
Iridium / Axially Chiral Phosphoramidite Complex Catalyzed Allylic Substitution of Allyl Carbonate Derivatives

In a nitrogen-filled atmosphere, [Ir(cod)Cl]2 (2.7 mg, 0.0040 mmol), the ligand (4.3 mg, 0.0080 mmol), KF (12 mg, 0.20 mmol), 18-crown-6 (53 mg, 0.20 mmol) and a Teflon-coated magnetic stirring bar are added to a 1-dram vial. Then, anhydrous THF (0.40 mL) is added. The mixture is stirred for 5 min. at ambient temperature before allyl carbonate derivative (0.20 mmol) and the silyl enolate derivative (0.40 mmol, 2.0 equiv) are added. The vial is sealed with a cap and stirred at 50 °C for 12 h. Then, water (1 mL) is added to the reaction mixture followed by 1 N HCl (0.5 mL). The resulting mixture is stirred vigorously for 3 h at room temperature. Brine (1 mL) and Et2O (1 mL) are added; the organic layer is separated, and the aqueous layer is extracted with Et2O (3 x 3 mL). The combined organic extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product is performed by flash column chromatography (gradient elution: hexane:Et2O = 50:1 to 5:1) to provide the product.
References
- Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones
Application
C-H Borylation of Arenes

Typical Procedure:
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
References
- Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate
Application
Iridium-catalyzed reaction of alcohols with enones affording 1,3-diketones
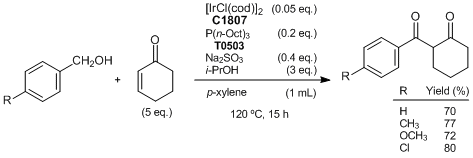
Typical procedure (R=H):
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
References
Application
Iridium-catalyzed alpha-alkylation of tert-butyl acetate

Typical procedure:
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
References
Application
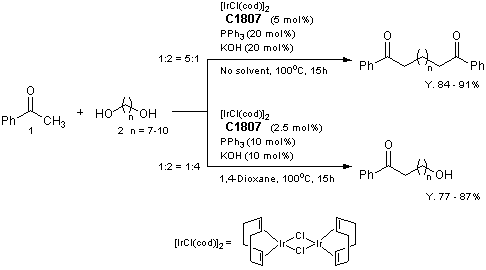
Selective Synthesis of Diketones and omega-Hydroxy Ketones by [IrCl(cod)]2/PPh3/KOH System
Reference
PubMed Literature
TCIMail
[Research Articles] Iridium-catalyzed reaction of alcohols with enones affording 1,3-diketones제품문서 (분석 차트를 제공하지 못할 수도 있으니 양해 바랍니다.)
SDS
언어를 선택하십시오.
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
샘플 시험성적서
시험성적서 샘플을 다운로드할 수 있습니다. 샘플은 반드시 최신 로트의 결과를 나타내는 것은 아닙니다.
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
죄송합니다만 찾으시는 분석차트는 없습니다.




