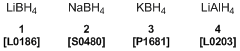A fuel cell is a kind of power generator which produces electricity by oxidizing hydrogen in oxygen or air. However, hydrogen is not easy to care for due to its being a very flammable and explosive gas.
Inorganic hydrides such as lithium borohydride (1), sodium borohydride (2), potassium borohydride (3), and lithium aluminum hydride (4) are relatively-stable crystals. The researchers have investigated fuel cells using them as hydrogen sources. For example, 2 reacts with water and generates hydrogen according to Scheme 1. But hydrogen generation is inhibited in alkaline aqueous solution, and can be regulated by using proper catalysts. In this case, the electrode reaction is represented by Scheme 2 and is able to extract electricity safely.
Maintenance Notice (3:15 - 7:30 AM February 22, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Notice of Discontinuing the Use of Password-Protected Compressed Files | Product Document Searching Made Easy by 2D Code! | TCI Life Science News February 2025 | [Product Highlights] Chemiluminescent Reagents for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)