Maximum quantity allowed is 999
Please select the quantity
Useful N-Heterocyclic Carbene Precatalysts “Bode Catalysts”
No.150(December 2012)

Recently, there have been many reports on carbon-carbon bond forming processes mediated by N-heterocyclic carbene (NHC) catalysts. Bode et al. have reported the high-yield and highly enantioselective synthesis of heterocyclic compounds using an NHC precatalyst 2. For example, an NHC in situ generated from 2 catalyzes the inverse electron demand Diels–Alder reaction of activated α,β-unsaturated aldehydes with α,β-unsaturated imines to afford the dihydropyridinones with remarkable enantioselectivities.1a)
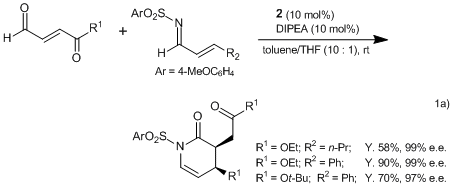
In case of using α-chloroaldehydes and α,β-unsaturated ketones, the oxodiene Diels–Alder reaction proceeds to afford the desired products with excellent enatioselectivities, with no more than 0.5 mol% of the catalyst.1b)

In addition, an achiral precatalyst 3 is also a useful precursor for the NHC-catalyzed redox esterification2a) and amidation2b) of α-functionalized aldehydes.
*For easier handling, 3 is sold as a perchlorate salt, instead of a chloride salt as reported in the literatures.

References
- 1)Highly enantioselective Diels–Alder reactions using Bode catalysts
- 2)N-Heterocyclic carbene-catalyzed esterification and amidation
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.