Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
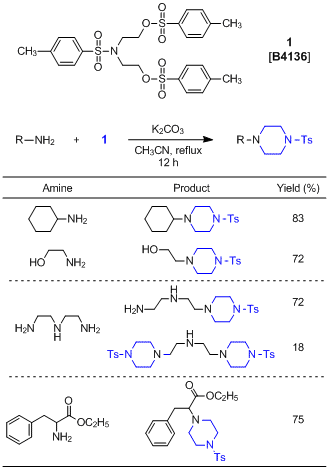
A Useful Reagent for the Synthesis of Piperazines
N,N-Bis[2-(p-tolylsulfonyloxy)ethyl]-p-toluenesulfonamide (1) is generally used for the synthesis of azacrown ethers and azamacrocyclic compounds. Recently, Huang and Li et al. have developed the method for the synthesis of piperazine blocks by the reaction of 1 with amines. In this reaction, N-alkyl-N’-tosylpiperazine derivatives are obtained by using aliphatic primary amines. When using aminoalcohols, 1 reacts with only the amino group to afford piperazines having a hydroxyl group at their terminal. Furthermore, the reaction of 1 with diamines or polyamines preferentially affords mono-piperazines with the formation of di-piperazines as minor products. Primary amines preferentially react with 1 compared with secondary amines due to having a higher reaction rate. It is presumed by means of DFT calculations that this reaction proceeds via the 6-membered ring transition state.
Related Products
References
- One-Step Cyclization: Synthesis of N-Heteroalkyl-N′-tosylpiperazines