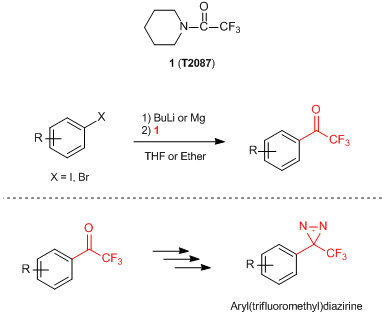
1-(Trifluoroacetyl)piperidine (1) has been used as a reagent for introducing a trifluoroacetyl group onto aromatic rings. Reactions of 1 with aryl-lithium species prepared by the halogen-lithium exchange of aryl halides or ortho-lithiation of aromatic compounds proceed smoothly under low-temperature, and afford the desired trifluoroacetylated compounds in good yields. When using aryl Grignard reagents, the same reaction proceeds but the yields of the products tend to decrease compared to that of the related aryl lithium species.
1 has been well used in the multi-step process to synthesize the (trifluoromethyl)diazirine moiety on aryl(trifluoromethyl)diazirines, which are efficient probes for photoaffinity labeling.
Maximum quantity allowed is 999
Please select the quantity
A Convenient Trifluoroacetylating Reagent
References
- Ginkgolide Derivatives for Photolabeling Studies: Preparation and Pharmacological Evaluation
- Synthesis and Biological Evaluation of Photoaffinity Labeled Fusidic Acid Analogues
- Comprehensive Synthesis of Photoreactive (3-Trifluoromethyl)diazirinyl Indole Derivatives from 5- and 6-Trifluoroacetylindoles for Photoaffinity Labeling