Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
Chemistry Chat
Keeping Up with Changes in Senior High School Chemistry
Hiroyuki Onuki
Toyo University Keihoku Senior High School
1. Introduction
In a previous report,1 I described how I encountered difficulties in conducting experiments in senior high school chemistry classes. In this article, I introduce the challenges of keeping up-to-date with the latest content in senior high school chemistry, which changes occasionally.
2. Changes in Terminology
Terminology changes over time. Based on the revisions in the Curriculum Guidelines in Japan announced in 2018,2 the terms used in Japanese textbooks have been significantly revised.3,4 Teachers must pay close attention to these changes to ensure they do not overlook them when revising the guidelines.
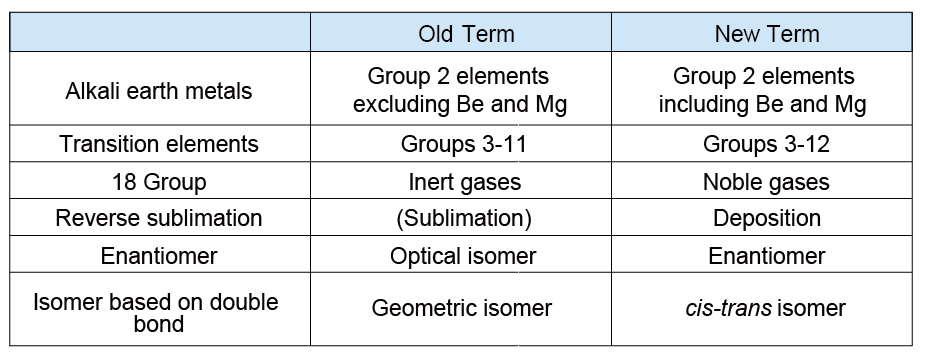
The teaching contents in schools are constantly changing. Hence, when I talk with people of different generations, It is interesting for me to note that they used outdated terms. For example, the terms "gram equivalent" and “normamality”: sound nostalgic.4,5
3. Thermochemistry
Japanese senior high school textbooks have historically described reactions involving heat exchange using thermochemical equations with an equals sign.6
For example, the reaction for the formation of water is expressed as:
H2 (g) + 1 2O2 (g) = H2O (l) + 286 kJ
However, from the fiscal year 2023, the textbooks have switched to thermochemical reactions that include enthalpy changes, and thus Japanese thermochemical equations are at last complied with international standards.7 The reaction of the formation of water is now expressed as:
H2 (g) + 1 2O2 (g) → H2O (l) ∆H = ‒286 kJ
This is one of the revisions of Japanese "Galápagosized" chemical education. The previous thermochemical equations focused on energy exchange viewing from an external system, whereas the new approach describes energy exchanges from internal system.
4. Experiment: Regulations of Chemicals
Several substances have become regulated in recent years because their hazards to humans and the environment have been revealed. Examples related to senior high school classes are given below.
4.1 Mercury
After the Minamata Convention on Mercury in 2013 came into effect, schools disposed of unnecessary equipment and reagents containing mercury.8 However, metallic mercury is indispensable for understanding the concept of pressure. The standard atmospheric pressure is described in textbooks as:
1.013 × 105 Pa = 760 mmHg = 1 atm
Unfortunately, many schools do not have sufficient quantities of metallic mercury, so students have to understand the relationship between atmospheric pressure and mercury in textbook only. Many students who graduated school without watching a Torricellian vacuum with their own eyes, which is a pity.
Other than metallic mercury, two mercury compounds appear in current textbooks in the following reactions:7
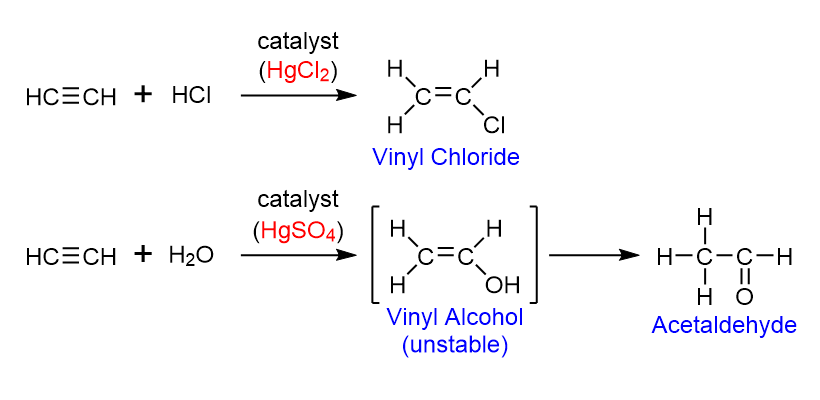
Mercury compounds play important roles as catalysts for addition reactions involving triple bonds, but these compounds can cause Minamata Disease, also called "methylmercury poisoning." It is quite difficult to make students understand the harmfulness of mercury-containing substances because we cannot show these reactions or demonstrate biological toxicity directly to them.
4.2 Benzene
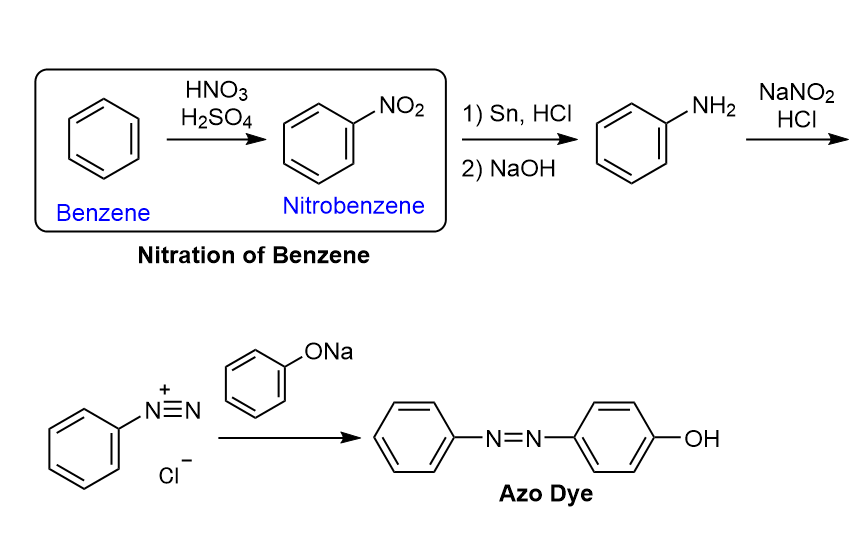
Benzene is carcinogenic.9 The classic experiment "Nitration of Benzene" was removed from textbooks revised in 2017. Although "Synthesis of Azo Dyes" remains, benzene is replaced by nitrobenzene as the starting material in the laboratory. It is a pity that students cannot directly handle the basic skeleton of aromatic compounds.
4.3 Carbon Tetrachloride
As an ozone-depleting substance,10 the use of carbon tetrachloride is limited. Students learn that its structure has a tetrahedral shape; four chloride atoms are arranged around the center carbon atom. Methane is introduced as a typical non-polar compound when studying molecular shapes and polarity. However, it is quite difficult to experimentally demonstrate that methane is non-polar because it is a gas at room temperature. Unlike methane, carbon tetrachloride is a liquid at room temperature, allowing students to visualize its non-polar properties through interactions with static electricity.11 The following is a demonstration of polarity:
Experiment: Produce a flow of water or carbon tetrachloride from a burette, and bring an electrically charged rod close to the flow.
Result: The polar water flow is bent by the electric charge, but the non-polar carbon tetrachloride flow is not.

Copyright by North Carolina School of Science and Mathematics, licensed under Creative Commons CC
5. Conclusion
Due to occasional changes in content and terminology definitions in senior high school chemistry, teachers must always keep up with the latest information. Also, we have to comply the legal regulation of substances handled and their effects on the environment. Furthermore, we should not forget to update our handouts/practice problems in classrooms and experimental procedures in laboratories.
References
- H. Onuki, TCIMAIL 2024, 195, 16. (English)
- Ministry of Education, Culture, Sports, Science and Technology, Commentary on the Curriculum Guidelines for Senior high schools, Science Edition, Math and Science Edition 2018, p. 103. (Japanese)
- Chemical Society of Japan, Subcommittee on Chemical Terminology, Chemistry and Education 2015, 63, 204; Chemical Society of Japan, Subcommittee on Chemical Terminology, Chemistry and Education 2016, 64, 92.
- Compendium of Terminology in Analytical Chemistry, IUPAC Orange Book, D. B. Hibbert ed., The Royal Society of Chemistry, 2022, Chapter 6.
- One gram equivalent is an acid (or base) mass that can produce 1 mol of hydrogen ions (hydroxide ions) in a neutralization reaction. The number of gram equivalents of acid or base dissolved in 1 L of solution is called that solution’s normality (symbol N). For example, one gram equivalent of sulfuric acid (molecular weight 98) is 49 g, and concentrated sulfuric acid (18 mol/L) is 36 N. This appeared in senior high school textbooks at least until 1983.
- K. Tatsumi et al., Ministry of Education, Culture, Sports, Science and Technology Approved Textbook "Chemistry", 2017, Suken Publishing. (Japanese)
- K. Tatsumi et al., Ministry of Education, Culture, Sports, Science and Technology Approved Textbook "Chemistry", 2022, Suken Publishing. (Japanese)
- Ministry of the Environment, "Please Cooperate with the Intensive Collection of Mercury-Containing Products" (Leaflet), 2018. (Japanese) (accessed 2024-6-20)
- International Labor Organization, International Chemical Safety Cards (ICSCs), (0015 Benzene), 2016. (accessed 2024-6-13)
- International Labor Organization, International Chemical Safety Cards (ICSCs), (0024 Carbon Tetrachloride) 2023. (accessed 2024-6-13)
- North Carolina School of Science and Mathematics, November. 12, 2011, Polarity Water and Carbon Tetrachloride, AP Chemistry, YouTube. (accessed 2024-6-12)
Author Information
Hiroyuki Onuki
His research interests are organic natural product chemistry, instrumental analyses, and chemical education.

