Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
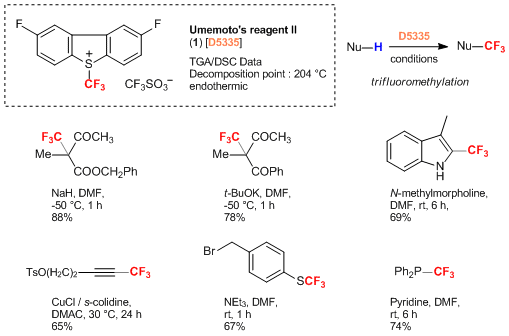
Umemoto’s Reagent II : A Powerful and Thermally Stable Trifluoromethylating Agent
Umemoto’s Reagent II (1), 2,8-diflouro-S-(trifluoromethyl)dibenzothiophenium salt has recently been reported as a new trifluoromethylating agent. Compared to Umemoto’s Reagent I (des-fluoro), Umemoto’s Reagent II shows superior thermal stability and electrophilicity. Under the basic condition, 1 smoothly reacts with β-dicarbonyl compounds to afford the corresponding trifluoromethylated products. The trifluoromethylation of 1 with terminal alkynes proceeds in the presence of a base and catalytic amounts of copper salts. Furthermore, 1 can be applied to the trifluoromethylation of various nucleophiles such as indoles, thiols and phosphines in the presence of base. 1 is a safe, solid trifluoromethylating agent that it is used for the synthesis of trifluoromethylated compounds which will attract attention in the pharmaceutical community.
References
- Powerful, Thermally Stable, One-Pot-Preparable, and Recyclable Electrophilic Trifluoromethylating Agents: 2,8-Difluoro- and 2,3,7,8-Tetrafluoro-S-(trifluoromethyl)dibenzothiophenium Salts