Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
The Laali Research Group at University of North Florida

Professor Kenneth Laali
Kenneth Laali is the Presidential Professor of Chemistry at University of North Florida. He completed his PhD in the UK at University of Manchester in 1977 and after postdoctoral stints in Europe at King’s College London, University of Strasbourg, University of Amsterdam, and at ETH-Zurich, he moved to University of Southern California in 1982 to work with Prof. George Olah. In 1985 he began his independent academic career at Kent State University in Ohio, where he rose through the ranks to become full professor in 1996. In 2009, he moved to UNF as a Professor/Founding Chair of Chemistry.
Prof. Laali’s research contributions in synthesis and NMR studies of carbocations and onium ions, and organofluorine and superacid chemistry have been internationally recognized. In the past decade, he has directed much of his attention to synthetic method development and catalysis in ionic liquids, and more recently to medicinal chemistry and drug discovery. Many of these projects involve organofluorine chemistry and benefit from extensive use of fluorinated compounds.
4-(Pentafluorosulfanyl)benzenediazonium Tetrafluoroborate
Abstract
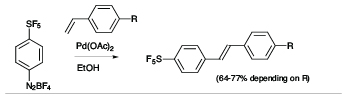
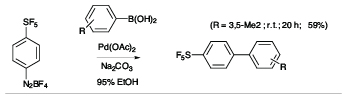
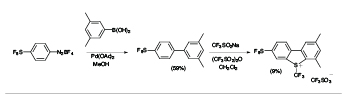
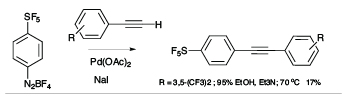
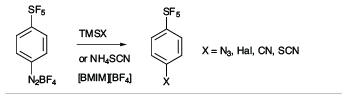
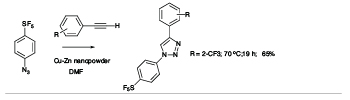
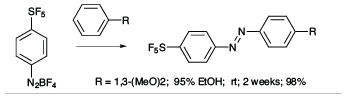
There is a high degree of interest in SF5-aromatics for potential applications in biomedical and materials field,[2] but lack of practical and safe methods that avoid the use of hazardous and exotic regents has hindered the development of synthetic methods capable of producing diverse libraries of SF5-bearing aromatics. In exploratory studies we showed that 4-(pentafluorosulfanyl)benzenediazonium tetrafluoroborate can act as a versatile launch pad for the synthesis of wide range SF5-aromatics employing diazonium ion chemistry, namely cross coupling, azo-coupling, homocoupling, dediazoniation and click chemistry, leading to highly diverse libraries of SF5-arenes as building blocks for further elaboration (see Scheme 1).[3]
Scheme 1

References
[1]See for example: Roglans, A.; Pla-Quintana, A.; M. Moreno-Manas Chem.Rev. 2006, 106, 4622-4643; Kuethe, J. T.; K. G. Childers Adv. Synth.Catal. 2008, 350, 1577-1586; Colleville, A. P.; Horan, R. A.; Tomkinson, N. C. O. Org. Process. Res. Dev. 2014, 18, 1128-1136; Li, X.; Yan, X.-Y.; Chang, H.-H.; Wang, L.-C.; Zhang, Y.; Chen, W.-W.; Li, Y.-W.; Wei, W.-L. Org.Biomol.Chem. 2012, 10, 495-497; Fabrizi, G.; Goggiamani, A.; Sferrazza, A.; Cacchi, S. Angew. Chem. Int. Ed. 2010, 49, 4067-4-070; Avila, C. M.; Patel, J. S.; Reddi, Y.; Saito, M.; Nelson, H. M.; Shunatona, H. P.; Sigman, M. S.; Sunoj, R. B.; Toste, F. D. Angew. Chem. Int. Ed. 2017, 56, 1-7; Barbero, M.; Cadamuro, S.; Dughera, S. Eur. J. Org. Chem. 2014, 598-605; Robinson, M. K.; Kochurina, V. S.; Hanna, J. M. Tetrahedron.Lett. 2007, 48, 7687-7690; Schmidt, B. Holter, F.; Berger, R.; Jessel, S. Adv. Synth. Catal. 2010, 352, 2463-2473.
[2]Altomonte, S.; Zanda, M. J. Fluorine. Chem. 2012, 143, 57-93; Savoie, P. R.; Welch, J. T. Chem. Rev. 2015, 115, 1130; Das, P.; Tokunaga, E.; Shibata, N. Tetrahedron.Lett. 2017, 58, 4803-4815
[3]Okazaki, T.; Laali, K. K.; Bunge, S. D.; Adas, S. K. Eur. J. Org. Chem. 2014, 1630-1644; Laali, K. K. e-EROS Encyclopedia of Reagents for Organic Synthesis 2016, 1-3; Okazaki, T.; Laali, K. K.; Reddy, A. S. J. Fluorine.Chem. 2014, 165, 91-95; Laali, K. K. US Patents 2016, 9,238,660 and 9,284,336; Snieckus, V.; Guimaraes, K. G. Synfacts, 2014, 10, 1134
Representative Examples