Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
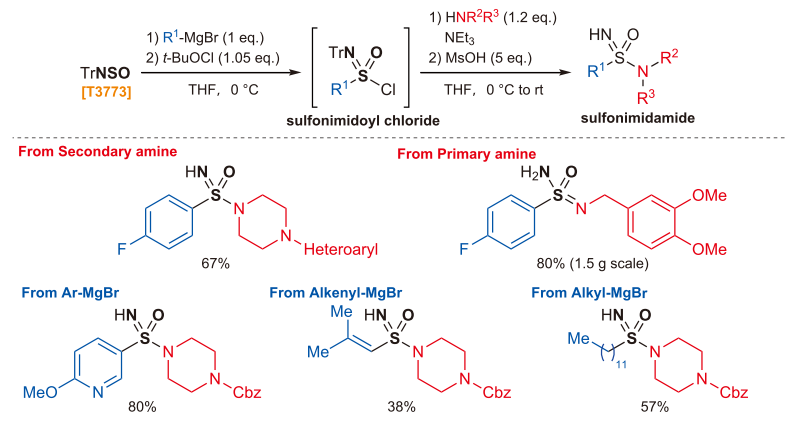
TrNSO (50.0 mg, 0.164 mmol) is dissolved in THF (1 mL). The reaction is cooled to 0 °C. Grignard reagent (0.164 mmol, 1.0 eq.) is added dropwise and the mixture is stirred for 5 min. tert-Butyl hypochlorite (19.5 µL, 0.172 mmol, 1.05 eq.) is added in the dark and the mixture is stirred for 15 min prior to addition of triethylamine (23 µL, 0.164 mmol, 1.0 eq.) and the corresponding amine (1.0-1.2 eq.). The reaction mixture is stirred at room temperature for 16 h. Methanesulfonic acid (53 µL, 0.819 mmol, 5.0 eq.) is added and the reaction solution is stirred vigorously for 15 min at room temperature before dilution with CH2Cl2. The solution is washed with saturated aqueous sodium bicarbonate solution, then the layers are separated and the aqueous phase is extracted three times with CH2Cl2. The combined organic layers are concentrated in vacuo. Purification by silica gel flash chromatography affords the desired sulfonimidamide.