Maximum quantity allowed is 999
CAS RN: 361447-81-6 | Product Number: P2822
1-Phenyl-1H-imidazol-3-ium Trifluoromethanesulfonate

Purity: >98.0%(HPLC)
- 1-Phenyl-1H-imidazol-3-ium Triflate
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 5G |
$142.00
|
9 | 2 | Contact Us | |
| 25G |
$459.00
|
1 | 2 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | P2822 |
| Purity / Analysis Method | >98.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__1__0H__9F__3N__2O__3S = 294.25 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
| CAS RN | 361447-81-6 |
| Reaxys Registry Number | 15506416 |
| PubChem Substance ID | 468592743 |
| MDL Number | MFCD32632561 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Melting point | 126.0 to 131.0 °C |
| NMR | confirm to structure |
| Melting Point | 130 °C |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| H.S.code* | 2933.29-000 |

-
Used Chemicals
-
Procedure
-
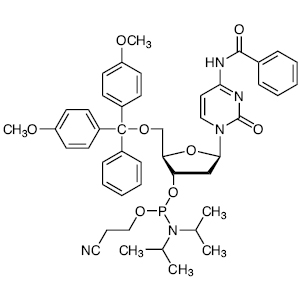
To an acetonitrile (2 mL) solution of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphordiamidite (0.42 g, 1.4 mmol) and N-PhIMT (0.34 g, 1.2 mmol) were added a DMF solution of 1 (0.40 g, 1.2 mmol) under nitrogen atmosphere. The reaction mixture was stirred overnight at room temperature, then diluted with TBME and washed with H2O, DMF:H2O (1:1) solution, H2O and brine. The organic layer was concentrated under reduced pressure. The resulting crude product was purified by column chromatography (hexane:ethyl acetate:triethylamine = 1:2:0.03 on silica gel) to give 2 as a white powder (0.43 g, 66% yield).
-
Experimenter’s Comments
-
The reaction mixtures were monitored by LCMS.
-
Analytical Data
-
Compound 2
1H NMR (400 MHz, DMSO-d6); δ 11.36 (brs, 1H), 8.05-7.96 (m, 2H), 7.73-7.64 (m, 1H), 7.59-7.51 (m, 2H), 7.45-7.37 (m, 1H), 6.25-6.17 (m, 1H), 4.72-4.62 (m, 1H), 4.62-4.52 (m, 1H), 4.51-4.38 (m, 1H), 4.32-4.15 (m, 1H), 3.84-3.66 (m, 2H), 3.66-3.51 (m, 2H), 2.84-2.73 (m, 2H), 2.47-2.22 (m, 2H), 1.67-1.53 (m, 3H), 1.27-1.03 (m, 12H).
13C NMR (101 MHz, DMSO-d6); δ 165.5, 163.6, 150.4, 135.8, 133.7, 129.3 (3C), 128.9 (2C), 119.0, 109.9, 84.1, 82.8, 72.8, 64.9, 58.3 (2C), 42.7, 37.7, 24.3 (4C), 19.8, 11.9.
31P NMR (162 MHz, DMSO-d6); δ 147.89.
-
Lead Reference
-
- Acid/Azole Complexes as Highly Effective Promoters in the Synthesis of DNA and RNA Oligomers via the Phosphoramidite Method
References
- N-phenylimidazolium triflate as a highly effective promoter for the interribonucleotide-bond formation via the phosphoramidite method
- Acid/Azole Complexes as Highly Effective Promoters in the Synthesis of DNA and RNA Oligomers via the Phosphoramidite Method
- Utility of azolium triflates as promoters for the condensation of a nucleoside phosphoramidite and a nucleoside in the Agrawal's stereoselective synthesis of nucleoside phosphorothioates
- Nucleosidic Phosphoramidite Synthesis via Phosphitylation: Activator Selection and Process Development
- Benzimidazolium triflate (a review)
Articles/Brochures
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.