Maintenance Notice (10:30 PM January 4 - 8:00 AM January 5, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197 | Notice of Discontinuing the Use of Password-Protected Compressed Files | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
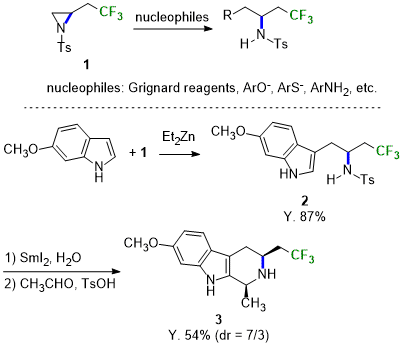
Aziridine Derivative for the Synthesis of a Trifluoroalkylamine Moiety
Sodeoka and Kawamura et al. have recently reported 1-tosyl-2-(2,2,2-trifluoroethyl)aziridine (1), which is utilized in the synthesis of trifluoroalkylamine derivatives. 1 reacts with various carbon and nitrogen nucleophiles to provide ring-opening adducts with high regioselectivity and in high yields. Furthermore, when the indole adduct 2 is treated with acetaldehyde, the tetrahydroharmine derivative containing a fluorinated functional group (3) is afforded via a Pictet-Spengler reaction. 1 is expected to be used as a building block for fluoroamine synthesis and heterocycles for pharmaceutical and agrochemical research.
Related Products
References
- N-Heterocycle-Forming Amino/Carboperfluoroalkylations of Aminoalkenes by Using Perfluoro Acid Anhydrides: Mechanistic Studies and Applications Directed Toward Perfluoroalkylated Compound Libraries