Maintenance Notice (10:30 PM January 4 - 8:00 AM January 5, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197 | Notice of Discontinuing the Use of Password-Protected Compressed Files | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
Aza-Cope Rearrangement
The aza-Cope rearrangement is one of the [3,3]-sigmatropic rearrangement reactions of nitrogen-substituted 1,5-hexadienes. The reaction mainly proceeds via a chair-like transition state as in the Cope rearrangement. The rearrangement can proceed regardless of the position of the nitrogen atom, and the 2-aza-Cope rearrangement is often utilized. The 3-aza-Cope rearrangement is the same as the aza-Claisen rearrangement and the 1-aza-Cope rearrangement is regarded as the retro-reaction of the aza-Claisen rearrangement. The aza-Cope rearrangement can be applied to the formation of seven-membered rings like the Cope rearrangement and many studies have been reported of the tandem type aza-Cope/Mannich reaction. Thanks to these reactions, heterocyclic compounds with fused-ring structures can be easily formed.
- Reagents:
- Acids
- Reactants:
- N-Substituted-1,5-hexadienes
- Products:
- N-Substituted-1,5-hexadienes
- Scheme:
-
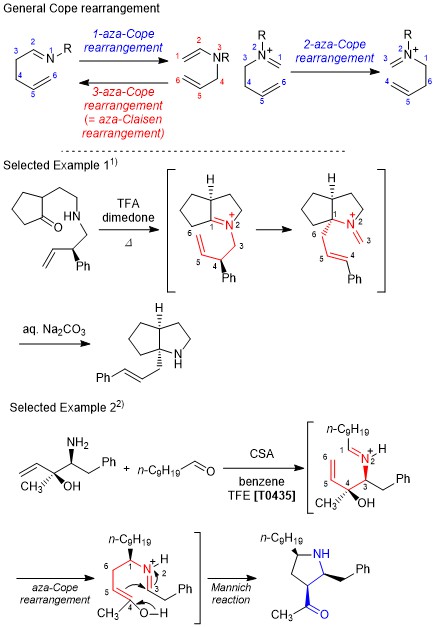
- Original literature:
-
- A Cleavage Reaction of α-Allylbenzylamines
- Review literature:
-
- The 1-aza-Cope rearrangement
-
- The Aza-Cope/Mannich Reaction
Related Name Eeactions
- Cope Rearrangement
- Oxy-Cope Rearrangement
- Claisen Rearrangement
- Mannich Reaction