Maximum quantity allowed is 999
Please select the quantity
CAS RN: 17455-13-9 | Product Number: C0860
18-Crown 6-Ether

Purity: >98.0%(GC)
Synonyms:
- 1,4,7,10,13,16-Hexaoxacyclooctadecane
Product Documents:
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | C0860 |
| Purity / Analysis Method | >98.0%(GC) |
| Molecular Formula / Molecular Weight | C__1__2H__2__4O__6 = 264.32 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic,Heat Sensitive |
| CAS RN | 17455-13-9 |
| Reaxys Registry Number | 1619616 |
| PubChem Substance ID | 87565919 |
| SDBS (AIST Spectral DB) | 5899 |
| Merck Index (14) | 2602 |
| MDL Number | MFCD00005113 |
Specifications
| Appearance | White or Colorless to Almost white or Almost colorless powder to lump to clear liquid |
| Purity(GC) | min. 98.0 % |
| Melting point | 39.0 to 43.0 °C |
| Solubility in Methanol | almost transparency |
Properties (reference)
| Melting Point | 40 °C |
| Boiling Point | 190 °C/0.7 mmHg |
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H302 : Harmful if swallowed. H319 : Causes serious eye irritation. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P280 : Wear eye protection/ face protection. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. |
Related Laws:
| RTECS# | MP4500000 |
Transport Information:
| H.S.code* | 2932.99-000 |
Application
Iridium / Axially Chiral Phosphoramidite Complex Catalyzed Allylic Substitution of Allyl Carbonate Derivatives
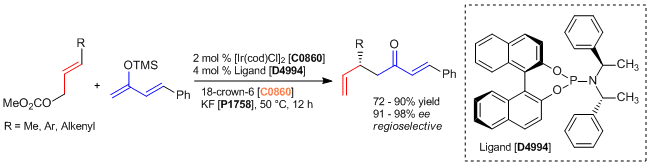
In a nitrogen-filled atmosphere, [Ir(cod)Cl]2 (2.7 mg, 0.0040 mmol), the ligand (4.3 mg, 0.0080 mmol), KF (12 mg, 0.20 mmol), 18-crown-6 (53 mg, 0.20 mmol) and a Teflon-coated magnetic stirring bar are added to a 1-dram vial. Then, anhydrous THF (0.40 mL) is added. The mixture is stirred for 5 min. at ambient temperature before allyl carbonate derivative (0.20 mmol) and the silyl enolate derivative (0.40 mmol, 2.0 equiv) are added. The vial is sealed with a cap and stirred at 50 °C for 12 h. Then, water (1 mL) is added to the reaction mixture followed by 1 N HCl (0.5 mL). The resulting mixture is stirred vigorously for 3 h at room temperature. Brine (1 mL) and Et2O (1 mL) are added; the organic layer is separated, and the aqueous layer is extracted with Et2O (3 x 3 mL). The combined organic extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product is performed by flash column chromatography (gradient elution: hexane:Et2O = 50:1 to 5:1) to provide the product.
References
- Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




