Maximum quantity allowed is 999
CAS RN: 53199-31-8 | Product Number: B3161
Bis(tri-tert-butylphosphine)palladium(0)
Purity: >98.0%(T)
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 250MG |
$100.00
|
0 | 0 | Contact Us | |
| 1G |
$286.00
|
7 | 0 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | B3161 |
Purity / Analysis Method 
|
>98.0%(T) |
| Molecular Formula / Molecular Weight | C__2__4H__5__4P__2Pd = 511.06 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Frozen (<0°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Heat Sensitive |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 53199-31-8 |
| Reaxys Registry Number | 14300595 |
| PubChem Substance ID | 87560386 |
| MDL Number | MFCD03094580 |
| Appearance | White to Yellow to Orange powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
| H.S.code* | 2843.90-000 |
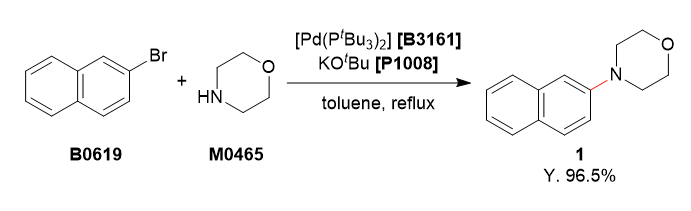
Used Chemicals
Procedure
To a 4-necked 200 mL flask was charged with 2-bromonaphthalene (5.20 g, 25.1 mmol, 1.0 equiv.), morpholine (3.27 mL, 37.5 mmol, 1.5 equiv.) and degassed toluene (75 mL). To this solution was added bis(tri-tert-butylphosphine)palladium(0) (256 mg, 0.501 mmol, 2.0 mol%) and potassium tert-butoxide (4.21 g, 37.5 mmol, 1.5 equiv.). The reaction mixture was refluxed for 3 h under argon atmosphere. The reaction mixture was cooled to room temperature and washed with water (50 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (50 mL). The combined organic layers were washed with brine (50 mL), dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (eluent: hexane/ethyl acetate, 85/15→70/30) to obtain 1 as a white solid (5.17 g, 96.5%).
Experimenter's Comments
The reaction mixture was monitored by TLC (hexane/ethyl acetate = 9/1, Rf = 0.70).
Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.76–7.69 (m, 3H), 7.42 (t, J = 7.2 Hz, 1H), 7.31 (t, J = 6.8 Hz, 1H), 7.25–7.28 (m, 1H), 7.13 (s, 1H), 3.92 (t, J = 4.8 Hz, 2H), 3.27 (t, J = 4.8 Hz, 2H).
13C NMR (101 MHz, CDCl3); δ 129.0, 127.6, 127.0, 126.5, 123.7, 119.1, 110.3, 67.1, 50.0.
Lead Reference
- Heterogeneous Rhodium‐Catalyzed Aerobic Oxidative Dehydrogenative Cross‐Coupling: Nonsymmetrical Biaryl Amines
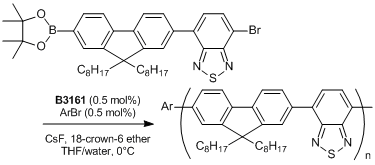
References
- Chain-Growth Suzuki Polymerization of n-Type Fluorene Copolymers
- E. Elmalem, A. Kiriy, W. T. S. Huck, Macromolecules 2011, 44, 9057.


Typical Procedure: An ampoule is charged with the thiocarbamate (0.442 mmol) and anhydrous degassed toluene (4 mL) is added via gastight syringe under nitrogen with stirring. A J. Youngs valve is fitted and the ampoule is placed into an oil bath pre-equilibrated at 100℃. After ca. 5 minutes the valve is removed and Pd(t-Bu3P)2 (2 mol%, 0.00884 mmol) is added as a solid, the valve is then replaced and the reaction mixture heated for 2.5 hours. A sample of the reaction mixture is removed via gastight syringe and found to contain the desired product (>99%).
Reference
- The Newman–Kwart Rearrangement of O‐Aryl Thiocarbamates: Substantial Reduction in Reaction Temperatures through Palladium Catalysis
Documents
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts

The requested analytical chart is not available. Sorry for the inconvenience.




