Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Please select the quantity
Highly Effective Iodinating Reagent
No.144(March 2011)
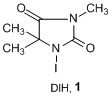
1,3-Diodo-5,5’-dimethylhidantoin (DIH, 1), which was first reported by Orazi, is an useful iodinating reagent.1) 1 has higher reactivity and selectivity than molecular iodine or N-iodosuccinimide (NIS), which are frequently used for iodination reactions. 1 is a pale yellow solid that does not sublime like molecular iodine, and has low toxicity, which makes it easier to handle. In addition, dimethylhidantoin, which is formed after the reaction, can easily be removed by aqueous extraction.
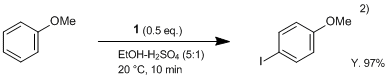
1 reacts smoothly at room temperature with aromatic compounds in the presence of sulfuric acid to give the corresponding iodinate in a high regioselectivity and a high yield. Moreover, even low-activated substances such as nitrobenzene can easily be iodinated by using sulfuric acid as solvent.2)
Togo et al. have reported an efficient method for preparing nitriles.3) According to their results, primary alcohols, and primary, secondary, and tertiary amines can be easily and efficiently converted into the corresponding nitriles in aqueous ammonia using 1.

Moreover, Togo et al. have also reported the efficient synthesis of 2-substituted-2-oxazolines by the reaction of 2-aminoethanol and aldehydes using 1.4) Moreover, it is notable that chiral bis-2-oxazolines, which are quite useful as chiral ligands in asymmetric synthesis, can also be prepared in moderate yields with chiral 2-phenylglycinol under the same conditions.
Typical Procedure: 2)
Sulfuric acid (2 mL) is added under stirring and cooling to a solution of anisole (1.1 g, 10 mmol) in ethanol (15 mL), and DIH (1; 1.9 g, 5 mmol) is then added in 3 portions over a period of 2-3 min. When the reaction is complete, the mixture is diluted with 3% solution of Na2SO3 (50 mL), and the precipitate is filtered off, washed with water, dried, and recrystallized from appropriate solvent. Yield of 4-iodoanisole 97%.
Sulfuric acid (2 mL) is added under stirring and cooling to a solution of anisole (1.1 g, 10 mmol) in ethanol (15 mL), and DIH (1; 1.9 g, 5 mmol) is then added in 3 portions over a period of 2-3 min. When the reaction is complete, the mixture is diluted with 3% solution of Na2SO3 (50 mL), and the precipitate is filtered off, washed with water, dried, and recrystallized from appropriate solvent. Yield of 4-iodoanisole 97%.
References
- 1) O. O. Orazi, R. A. Corral, H. E. Bertorello, J. Org. Chem. 1965, 30, 1101.

- 2) V. K. Chaikovskii, V. D. Filimonov, A. A. Funk, V. I. Skorokhodov, V. D. Ogorodnikov, Russ. J. Org. Chem. 2007, 43, 1291.

- 3) M. Ishihara, H. Togo, Tetrahedron 2007, 63, 1474.
 ; S. Iida, H. Togo, Tetrahedron 2007, 63, 8274.
; S. Iida, H. Togo, Tetrahedron 2007, 63, 8274.
- 4) S. Takahashi, H. Togo, Synthesis 2009, 2329.

Related Compounds
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

