Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
CAS RN: 81290-20-2 | Product Number: T1570
(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]
![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] No-Image](/medias/T1570.jpg?context=bWFzdGVyfHJvb3R8NzU0MTB8aW1hZ2UvanBlZ3xhRGRsTDJoaVpTODRPVEl6TkRFM05qY3pOelU0TDFReE5UY3dMbXB3Wnd8YjdiMmNiNWZiODExMzE5ZDE3Mzg5MDVmNDYwOGIzYTM1OGQxYzY1YWZhZTlkNTg3OTczMmI0MmY2YzhhNTQzZg)
Purity: >97.0%(GC)
- (Trimethylsilyl)trifluoromethane
- Ruppert-Prakash Reagent
| Size | Unit Price | Philadelphia, PA | Portland, OR | Japan* | Quantity |
|---|---|---|---|---|---|
| 5G |
$34.00
|
8 | 1 | ≥40 |
|
| 25G |
$91.00
|
4 | 4 | ≥100 |
|
* Items in stock locally ship in 1-2 business days. Items from Japan stock are able to ship from a US warehouse within 2 weeks. Please contact TCI for lead times on items not in stock. Excludes regulated items and items that ship on ice.
* To send your quote request for bulk quantities, please click on the "Request Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Product Number | T1570 |
| Purity / Analysis Method | >97.0%(GC) |
| Molecular Formula / Molecular Weight | C__4H__9F__3Si = 142.20 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
| CAS RN | 81290-20-2 |
| Reaxys Registry Number | 4241868 |
| PubChem Substance ID | 87577328 |
| SDBS (AIST Spectral DB) | 19694 |
| Merck Index (14) | 9683 |
| MDL Number | MFCD00145454 |
| Appearance | Colorless to Light yellow clear liquid |
| Purity(GC) | min. 97.0 % |
| Boiling Point | 55 °C |
| Flash point | -32 °C |
| Specific Gravity (20/20) | 0.96 |
| Refractive Index | 1.33 |
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H225 : Highly flammable liquid and vapor. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P240 : Ground/bond container and receiving equipment. P210 : Keep away from heat/sparks/open flames/hot surfaces. No smoking. P233 : Keep container tightly closed. P243 : Take precautionary measures against static discharge. P241 : Use explosion-proof electrical/ ventilating/ lighting/ equipment. P242 : Use only non-sparking tools. P280 : Wear protective gloves/ eye protection/ face protection. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P403 + P235 : Store in a well-ventilated place. Keep cool. |
| UN Number (DOT-AIR) | UN1993 |
| Class (DOT-AIR) | 3 |
| Packing Group (TCI-A) | II |
| HS Number | 2931.90.9010 |
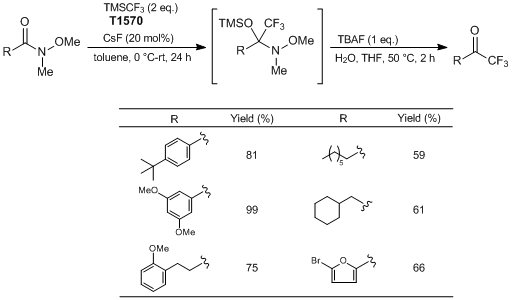
To a 50 mL round bottom flask equipped with a stir bar is added CsF (0.1512 g, 1.0 mmol, 0.2 eq.). Toluene (2.5 mL) is added to the flask, followed by 4-(tert-butyl)-N-methoxy-N-methylbenzamide (1.11 g, 5.0 mmol, 1 eq.). The flask is sealed with a septum equipped with an inlet needle as an exit valve. The flask is cooled to 0 °C for 10 minutes. Once cooled, TMSCF3 (1.42 g, 10.0 mmol, 2 eq.) is added to the reaction mixture dropwise over a period of ≈ 10 minutes. After completion of addition, the reaction mixture is allowed to stir at 0 °C for 10 minutes. The cooling bath is removed and the reaction mixture is allowed to stir at room temperature overnight. (Upon reaching room temperature, the reaction occurs and is mildly exothermic and gas is evolved.) Reaction progress is monitored by 1H NMR.
Once complete conversion to the silylated intermediate is confirmed, water (5 mL) followed by TBAF (5 mL, 1 M in THF, 1 eq.) is added to the reaction flask. The flask is equipped with a reflux condenser, open to air. The contents are then heated to 50 °C, and allowed to stir at that temperature for 2 hours. Once cooled to room temperature, the reaction mixture is diluted with Et2O (≈ 30 mL), and transferred to a separatory funnel. The organic layer is washed with deionized water (3 × 30 mL), followed with a brine solution (1 × 30 mL). The organic layer is dried with Na2SO4 and the solvent is removed in vacuo by rotary evaporation to yield crude trifluoromethylketone. Further purification is accomplished by flash chromatography (hexane : EtOAc = 8 : 2) to produce the pure CF3 ketone as an orange solid (0.935 g, Y. 81%).
References

Reference

Reference
- Synthesis of gem-difluorinated cyclopropanes and cyclopropenes: trifluoromethyltrimethylsilane as a difluorocarbene source
Articles/Brochures
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.



![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] (Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

