Maximum quantity allowed is 999
请选择数量
Asymmetric Two-center Phase-transfer Catalyst
No.135(July 2008)
- D3476
- (S,S)-TaDiAS-2nd [=6,10-Dibenzyl-N,N'-dimethyl-N,N,N',N'-tetrakis(4-methylbenzyl)-1,4-dioxaspiro[4.5]decane-(2S,3S)-diylbis(methylammonium) Tetrafluoroborate] (1a)
- D3475
- (R,R)-TaDiAS-2nd [=6,10-Dibenzyl-N,N'-dimethyl-N,N,N',N'-tetrakis(4-methylbenzyl)-1,4-dioxaspiro[4.5]decane-(2R,3R)-diylbis(methylammonium) Tetrafluoroborate] (1b)

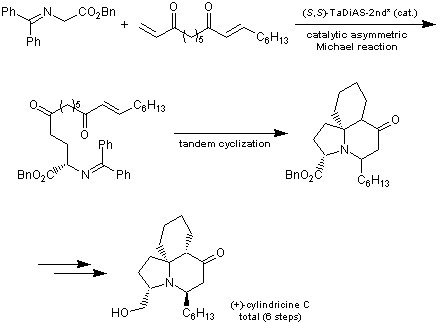
TaDiAS (Tartrate-derived Diammonium Salt) is an asymmetric phase transfer catalyst developed by Shibasaki et al. TaDiAS has two ammonium ions in the molecule and its ions cooperatively act together to hold anions in an asymmetric space. Previously, TaDiAS has been achieved in high asymmetric yields. More recently, TaDiAS-2nd has been developed in which an alkyl chain of the acetal site of TaDiAS is substituted with a cyclohexane ring in order to improve the reactivity and enantioselectivity of these catalysts. Tandem synthesis of alkaloid (+)-Cylindricine C has been reported using TaDiAS-2nd as an asymmetric Michael reaction catalyst.
* In the literature, arrangement of two benzyl groups in TaDiAS-2nd is in the (S,S)-form. However, our product here is a mixture of diastereomers. Although there may be slight differences in their reactivity and enantioselectivity, it is confirmed that the reaction proceeds without any problem using our product.
References
- 1)Short synthesis of (+)-cylindricine C by using TaDiAS-2nd
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.