Maximum quantity allowed is 999
请选择数量
Diamidite for the Synthesis of Phosphodiesters
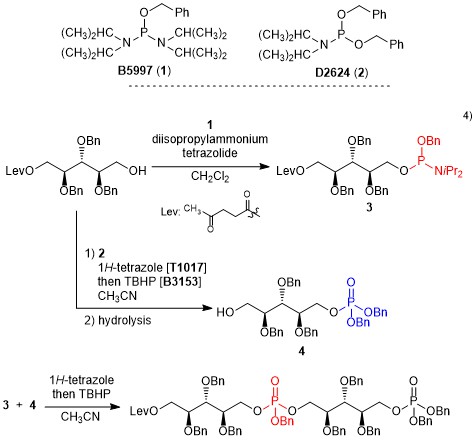
Benzyl N,N,N',N'-tetraisopropylphosphorodiamidite (1) is reportedly used in the synthesis of phosphodiesters1) and phosphorylation of inositols2) and nucleosides.3) 1 promptly reacts with alcohols in the presence of a base like 1H-tetrazole to afford a phosphoramidite. Since phosphoramidite can react with another alcohol, a phosphite trimester with different alcohol species can be synthesized. Successive oxidation of the phosphorus atom and deprotection gives a phosphodiester. Whereas, dibenzyl N,N-diisopropylphosphoramidite (2) reacts with only one alcohol molecule, phosphate monoesters can be synthesized in a same manner. Thus, 1 and 2 are properly used in a synthesis of phosphate esters. For instance, Stehle and Peschel et al. have synthesized the ribitol phosphate dimer by using 1 and 2.4)
Related Products
- B5997
- Benzyl N,N,N',N'-Tetraisopropylphosphorodiamidite
- D2624
- Dibenzyl N,N-Diisopropylphosphoramidite
References
- 1) A Simple and Effective Chemical Phosphorylation Procedure for Biomolecules
- 2) Synthetic Approaches to Heavily Lipidated Phosphoglyceroinositides
- 3) Transition State-Based Sialyltransferase Inhibitors: Mimicking Oxocarbenium Ion by Simple Amide
- 4) Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity