Maximum quantity allowed is 999
请选择数量
CAS RN: 1109-15-5 | 產品號碼: T2313
Tris(pentafluorophenyl)borane
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
產品規格
| Appearance | White to Gray to Brown powder to crystal |
| Purity(NMR) | min. 98.0 atom% |
| Water | max. 1.0 % |
性質
| 熔點 | 128 °C |
| Maximum Wavelength | 306 nm (Toluene) |
| 溶解性(可溶於) | Toluene |
GHS
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相關法規
運輸資料
| HS編碼* | 2931.90-000 |
Application
Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane
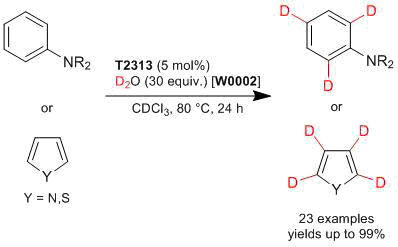
Experimental procedure: N,N-Dibenzylaniline (140 mg, 0.513 mmol), tris(pentafluorophenyl)borane (17.0 mg, 0.033 mmol), D2O (306 mg, 15.3 mmol) are placed in a sealed tube. The reaction mixture is stirred at 80 °C for 24 h. After the reaction is completed, the reaction mixture is purified by silica gel column chromatography (cyclohexane:ethylacetate = 20:1) to afford N,N-dibenzylaniline-2,4,6-d3 (134 mg) in 95% yield.
References
- B(C6F5)3‑Catalyzed Regioselective Deuteration of Electron-Rich Aromatic and Heteroaromatic Compounds
Application
High Throughput Sequence-controlled Oligosiloxane Synthesis

References
- By-Product-Free Siloxane-Bond Formation and Programmed One-Pot Oligosiloxane Synthesis
Application
Frustrated Lewis Pair (FLP)-induced Hydrogenations of Silyl Enol Ethers

References
- Heterolytic dihydrogen activation with the 1,8-bis(diphenylphosphino)naphthalene/B(C6F5)3 pair and its application for metal-free catalytic hydrogenation of silyl enol ether
Application
Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)

Synthesis of multisubstituted silanes:
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
References
- Formal SiH4 chemistry using stable and easy-to-handle surrogates
Application
Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)

Typical procedure (R, R’ = n-Pr): 4-Heptanone (1.00 g, 8.76 mmol) is weighed into a 125 mL reactor. Subsequently, B(C6F5)3 (0.224 g, 0.43 mmol) dissolved in Et2O (14.3 mg, 20 mL, 0.19 mol) is added to the reactor. The reactor is sealed and attached to a hydrogen gas line. The flask is purged ten times at 15 atm with hydrogen gas. The reactor is then pressurized with 60 atm hydrogen gas and placed in an oil bath for 12 h at 70 °C. The reactor is slowly vented and all the volatiles are collected by vacuum distillation while cooling the collected distillate with liquid nitrogen. The solvent is removed by applying a gentle stream of N2 gas to give 4-heptanol (886 mg, 87% yield, and 99 % conversion determined by 1H NMR).
References
- T. Mahdi, D. W. Stephan, J. Am. Chem. Soc. 2014, 136, 15809.

- Highlighted in Chem. Eng. News 2014, 92 (44), 8.

Application
Metal-Free Hydrogenation of N-Phenyl Aromatic Rings

Typical procedure (Entry 1): A 25 mL glass bomb equipped with Teflon screw cap is charged with a solution of B(C6F5)3 (37.9 mg, 1 eq.) and N-isopropylaniline (10.0 mg, 0.074 mmol) in toluene (1 mL). The reaction tube is degassed three times through a freeze-pump-thaw cycle on the vacuum/H2 line and filled with H2 (4 atm) at -196 °C. The reaction bomb is placed in a 110 °C oil bath for 36 h. The toluene is removed under reduced vacuum to yield a white precipitate. The product is washed with pentane (2 x 2 mL) and dried under reduced pressure to give [iPrNH2Cy][HB(C6F5)3] (93 %).
References
- 1)T. Mahdi, Z. M. Heiden, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 4088.

- 2)D. W. Stephan, G. Erker, Angew. Chem., Int. Ed. 2010, 49, 46.

Application
Metal-free Hydrogenation of Imines Catalyzed by B(C6F5)3

Typical Procedure: In a glovebox, B(C6F5)3 (18.2 mg, 0.0355 mmol, 20 mol%) is dissolved in dry diisopropylamine (2.5 mL, 1.8 g, 17 mmol) and the solution is added to N-benzylidene-tert-butylamine (28.7 mg, 0.177 mmol, 1 eq.). The resulting solution is transferred to a 25 mL bomb with a sealable Teflon tape and magnetic stirbar. The reaction vessel is sealed, removed from the glovebox and stirred at 100°C for 24 h after which it is cooled to room temperature. The reaction mixture is quenched by the addition of silica followed by elution through a short silica column. The filtrate is concentrated in vacuo to give the desired product.
References
PubMed Literature
產品介紹報導
[Product Highlights] Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane[Product Highlights] A Bisphosphine Usable for Metal-free Hydrogenations
[Research Articles] High Throughput Sequence-controlled Oligosiloxane Synthesis
[Research Articles] Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)
[Research Articles] Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)
[Research Articles] Metal-Free Hydrogenation of N-Phenyl Aromatic Rings
[Research Articles] Metal-Free Hydrogenation of Imines Catalyzed by B(C6F5)3
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜

請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。





