Maintenance Notice (10:30 PM January 4 - 8:00 AM January 5, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197 | Notice of Discontinuing the Use of Password-Protected Compressed Files | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
请选择数量
CAS RN: 14024-18-1 | 產品號碼: I0079
Tris(2,4-pentanedionato)iron(III)

* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
產品規格
| Appearance | Red to Dark red to Brown powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
性質
| 熔點 | 185 °C(dec.) |
| 水溶性 | Slightly soluble |
| 溶解性(可溶於) | Ethanol, Acetone, Benzene, dichloromethane, Toluene, Chloroform |
GHS
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H302 : Harmful if swallowed. H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. |
相關法規
| RTECS # | NO8960000 |
運輸資料
| HS編碼* | 2914.19-000 |
Application
2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation

Reference
- Iron-Catalyzed Di?uoromethylation of Arylzincs with Di?uoromethyl 2?Pyridyl Sulfone
Application
Iron/dppbz-catalyzed Decarboxylative Couplings of Active Esters
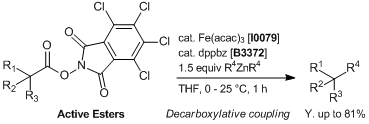
In a screwed-capped vial active ester (0.1 mmol), Fe(acac)3 (0.005 – 0.04 mmol) and dppbz (0.006 – 0.048 mmol) are weighed. The vial is then sealed, evacuated and back-filled with Ar. Then, 0.5 mL of distilled THF is added. The mixture is stirred for 5 minutes under Ar and diarylzinc reagent in THF (ca. 0.3 mol/L, 0.15 – 0.25 mmol) is added in one portion at 0 - 25 °C to the mixture and stirred at the same temperature for 1 h. After this time, the reaction is quenched with 1 mol/L HClaq, sat. NH4Claq or H2O and diluted with diethyl ether. The organic layer is separated and dried over Na2SO4 anhydrous, filtered and evaporated to dryness. Pure products are obtained after column chromatography or preparative TLC.
References
Application
Iron-Catalyzed ortho-Alkylation of Carboxamides

Typical procedure (R = benzyl): To Fe(acac)3 (17.1 mg, 0.05 mmol), dppe (29.9 mg, 0.075 mmol), and 3-methyl-N-(quinolin-8-yl)benzamide (131 mg, 0.5 mmol) are added THF (4 mL), and benzyl chloride (190 mg, 1.5 mmol). The mixture is then placed in an oil bath at 65 °C and PhMgBr (16% in THF, ca. 1 mol/L) (0.6 mL, 0.6 mmol) is added in a single portion. Upon completion of PhMgBr addition, the reaction mixture is stirred at 65 °C for 1 min. Then PhMgBr (16% in THF, ca. 1 mol/L) (1.3 mL, 1.3 mmol) is added dropwise at 65 °C for 9 min. The reaction mixture is stirred at 65 °C for 1 min and brought to rt. Subsequently the reaction mixture is quenched with a saturated solution of ammonium chloride (535 mg, 0.01 mol) and extracted with dichloromethane (3 x 3 mL). The combined organic extracts are dried over MgSO4, filtered and concentrated to give a residue. The residue is purified by silica gel flash chromatography (eluent: hexanes/EtOAc = 20:1) to give 2-benzyl-5-methyl-N-(quinolin-8-yl)benzamide (175 mg, 99% yield) as a white solid.
References
Application
Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
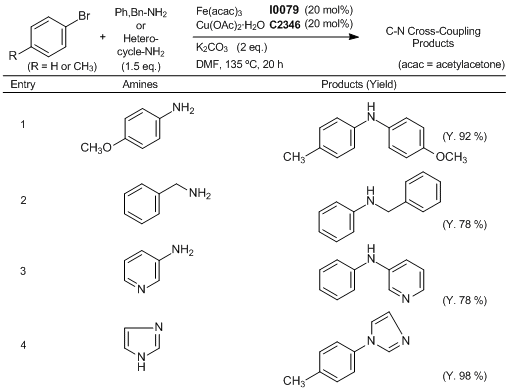
Typical procedure (Entry 1): A Schlenk tube is charged with p-anisidine (92.4 mg), K2CO3 (138 mg), Fe(acac)3 (34.4 mg), and Cu(OAc)2· H2O (20 mg). Under a nitrogen atmosphere, 4-bromotoluene (85.5 mg) is added followed by dry DMF (0.8 mL). The reaction mixture is then heated to 135 °C, and stirred for 20 h. The mixture is cooled to room temperature and diluted with diethyl ether. The resulting suspension is filtered through a celite pad and the filtrate is concentrated. The residue is purified by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to give 4-methoxy-N-p-tolylaniline (98.1 mg, 92 %).
References
Application
Iron-Catalyzed Cross-Coupling Reaction

Reference
考研文獻
TCIMail
[Product Highlights] 2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation[Research Articles] Fe or Ni-catalyzed Decarboxylative C-C Couplings of Active Esters
[Research Articles] Iron-Catalyzed ortho-Alkylation of Carboxamides
[Research Articles] Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜
請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。




