Maximum quantity allowed is 999
请选择数量
CAS RN: 52462-29-0 | 產品號碼: D2751
Dichloro(p-cymene)ruthenium(II) Dimer

* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | D2751 |
| 純度/分析方法 | >95.0%(T) |
| 分子式 / 分子量 | C__2__0H__2__8Cl__4Ru__2 = 612.38 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 52462-29-0 |
| PubChem Substance ID | 87569153 |
| MDL編號 | MFCD00064793 |
產品規格
| Appearance | Light yellow to Brown powder to crystal |
| Purity(Argentometric Titration) | min. 95.0 % |
性質
GHS
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. H412 : Harmful to aquatic life with long lasting effects. |
| 防範說明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P273 : Avoid release to the environment. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相關法規
運輸資料
| HS編碼* | 2843.90-000 |
Application
Ruthenium-catalyzed Direct C―H Amidation of Arenes

Typical Procedure:
Acetophenone (0.4 mmol), p-toluenesulfonyl azide (0.2 mmol), [RuCl2(p-cymene)]2 (4.9 mg, 4 mol%), AgNTf2 (12.5 mg, 16 mol%), NaOAc (3.3 mg, 20 mol%), and 1,2-dichloroethane (0.5 mL) are added to a screw-capped vial equipped with a spinvane triangular-shaped Teflon stirbar. The reaction mixture is stirred in a preheated oil bath at 80 °C for 12 h and then cooled to room temperature, filtered through a pad of Celite, and washed with ethyl acetate (10 mL×3). The filtrate is concentrated under reduced pressure and the residue is purified by chromatography on silica gel (n-hexane : EtOAc = 3 : 1) to give the desired product (56.1 mg, Y. 97%).
Acetophenone (0.4 mmol), p-toluenesulfonyl azide (0.2 mmol), [RuCl2(p-cymene)]2 (4.9 mg, 4 mol%), AgNTf2 (12.5 mg, 16 mol%), NaOAc (3.3 mg, 20 mol%), and 1,2-dichloroethane (0.5 mL) are added to a screw-capped vial equipped with a spinvane triangular-shaped Teflon stirbar. The reaction mixture is stirred in a preheated oil bath at 80 °C for 12 h and then cooled to room temperature, filtered through a pad of Celite, and washed with ethyl acetate (10 mL×3). The filtrate is concentrated under reduced pressure and the residue is purified by chromatography on silica gel (n-hexane : EtOAc = 3 : 1) to give the desired product (56.1 mg, Y. 97%).
References
Application
Ruthenium-Catalyzed Isoquinolone Synthesis
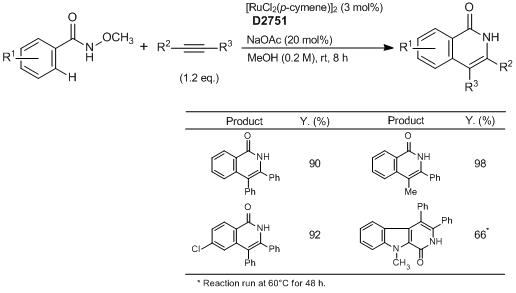
Typical Procedure:
A mixture of N-methoxybenzamide (0.50 mmol, 1.0 eq.), the alkyne (if solid) (0.60 mmol, 1.2 eq.), [RuCl2(p-cymene)]2 (9.2 mg, 0.015 mmol, 3.0 mol%) and NaOAc (8.2 mg, 0.10 mmol, 20.0 mol%) are placed in a Schlenk tube equipped with a stir bar. Dry MeOH (2.5 mL) is added (followed by the alkyne if it is a liquid) and the mixture is stirred at room temperature for 8 h under an argon atmosphere. Afterwards, the mixture is diluted with CH2Cl2 and transferred to a round-bottomed flask. Silica is added to the flask and volatiles are evaporated under reduced pressure. The purification is performed by flash column chromatography on silica gel (EtOAc : petroleum ether=1:5 to 1:1, typically) to give the corresponding pure products.
A mixture of N-methoxybenzamide (0.50 mmol, 1.0 eq.), the alkyne (if solid) (0.60 mmol, 1.2 eq.), [RuCl2(p-cymene)]2 (9.2 mg, 0.015 mmol, 3.0 mol%) and NaOAc (8.2 mg, 0.10 mmol, 20.0 mol%) are placed in a Schlenk tube equipped with a stir bar. Dry MeOH (2.5 mL) is added (followed by the alkyne if it is a liquid) and the mixture is stirred at room temperature for 8 h under an argon atmosphere. Afterwards, the mixture is diluted with CH2Cl2 and transferred to a round-bottomed flask. Silica is added to the flask and volatiles are evaporated under reduced pressure. The purification is performed by flash column chromatography on silica gel (EtOAc : petroleum ether=1:5 to 1:1, typically) to give the corresponding pure products.
References
考研文獻
TCIMail
[TCIMAIL No.107] Arene Ruthenium(II) Complexes[Research Articles] Ruthenium-catalyzed Direct C-H Amidation of Arenes
[Research Articles] Ruthenium-Catalyzed Isoquinolone Synthesis
產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜
請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。




