Maximum quantity allowed is 999
请选择数量
CAS RN: 12112-67-3 | 產品號碼: C1807
Chloro(1,5-cyclooctadiene)iridium(I) Dimer

* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | C1807 |
| 純度/分析方法 | >93.0%(T) |
| 分子式 / 分子量 | C__1__6H__2__4Cl__2Ir__2 = 671.70 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Refrigerated (0-10°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Air Sensitive,Heat Sensitive |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片), 250MG-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 12112-67-3 |
| Reaxys-RN | 14372408 |
| PubChem Substance ID | 87560730 |
| MDL編號 | MFCD00012414 |
產品規格
| Appearance | Orange to Amber to Dark red powder to crystal |
| Purity(Argentometric Titration) | min. 93.0 % |
性質
| 熔點 | 205 °C(dec.) |
| 水溶性 | Insoluble |
GHS
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相關法規
運輸資料
| HS編碼* | 2843.90-000 |
Application
Iridium / Axially Chiral Phosphoramidite Complex Catalyzed Allylic Substitution of Allyl Carbonate Derivatives

In a nitrogen-filled atmosphere, [Ir(cod)Cl]2 (2.7 mg, 0.0040 mmol), the ligand (4.3 mg, 0.0080 mmol), KF (12 mg, 0.20 mmol), 18-crown-6 (53 mg, 0.20 mmol) and a Teflon-coated magnetic stirring bar are added to a 1-dram vial. Then, anhydrous THF (0.40 mL) is added. The mixture is stirred for 5 min. at ambient temperature before allyl carbonate derivative (0.20 mmol) and the silyl enolate derivative (0.40 mmol, 2.0 equiv) are added. The vial is sealed with a cap and stirred at 50 °C for 12 h. Then, water (1 mL) is added to the reaction mixture followed by 1 N HCl (0.5 mL). The resulting mixture is stirred vigorously for 3 h at room temperature. Brine (1 mL) and Et2O (1 mL) are added; the organic layer is separated, and the aqueous layer is extracted with Et2O (3 x 3 mL). The combined organic extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product is performed by flash column chromatography (gradient elution: hexane:Et2O = 50:1 to 5:1) to provide the product.
References
- Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones
Application
C-H Borylation of Arenes

Typical Procedure:
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
A 25 mL-flask assembled a magnetic stirring bar, a septum inlet, and a condenser is charged with chloro(1,5-cyclooctadiene)iridium(I) dimer (10.1 mg, 0.015 mmol), 2,2’-bipyridyl (4.7 mg, 0.03 mmol), and bis-(pinacolato)diboron (254.0 mg, 1.0 mmol) and then flushed with nitrogen. An arene (60 mmol) is added, and the mixture is stirred at 80 °C for 16 h. The reaction mixture is analyzed by GC and GC mass spectroscopy. The product is extracted with benzene, washed with brine, and dried over MgSO4. Kugelrohr distillation gives analytically pure samples.
References
- Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate
Application
Iridium-catalyzed reaction of alcohols with enones affording 1,3-diketones
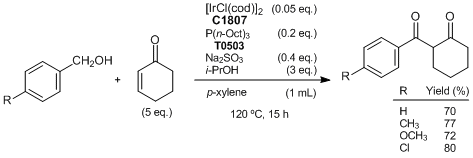
Typical procedure (R=H):
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
A mixture of [IrCl(cod)]2 (34 mg, 0.05 mmol), P(n-Oct)3 (74 mg, 0.2 mmol), i-PrOH (180 mg, 3 mmol), Na2SO3 (50 mg, 0.4 mmol), benzyl alcohol (108 mg, 1 mmol) and 2-cyclohexen-1-one (481 mg, 5 mmol) in p-xylene (1 mL) is stirred at 120 °C for 15 h under an argon atmosphere in a pressure tube. The product is purified by column chromatography on 230-400 mesh silica gel (eluent: hexane/ethyl acetate = 40/1) to give the desired 1,3-diketone (142 mg, 70% yield).
References
Application
Iridium-catalyzed alpha-alkylation of tert-butyl acetate

Typical procedure:
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
A mixture of [IrCl(cod)]2 (34 mg), PPh3 (39 mg), tert-BuOK (224 mg), n-butanol (74 mg) and tert-butyl acetate (449 mg) in tert-BuOH (1 mL) is stirred at 100 °C for 15 h under Ar. The product (tert-butyl hexanoate) is obtained in 74% yield (estimated using GC), and is isolated by column chromatography on 230-400 mesh silica-gel (eluent: hexane/THF = 50/1) in 62 % yield (107 mg).
References
Application
Selective Synthesis of Diketones and omega-Hydroxy Ketones by [IrCl(cod)]2/PPh3/KOH System
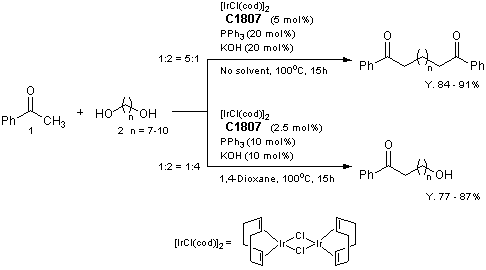
Reference
考研文獻
文章/手冊
TCIMail
[Research Articles] Iridium-catalyzed reaction of alcohols with enones affording 1,3-diketones產品文件 (部分產品的分析圖譜無法提供,敬請諒解。)
SDS
請選擇語言。
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
請輸入批號
輸入的批號不正確
示例 CoA
可下載CoA示例。注:該示例可能非最新批次的CoA。
目前沒有該產品的 CoA 示例。
分析圖譜
請輸入批號
輸入的批號不正確
很抱歉,您搜索的分析圖譜無法提供。




