Maximum quantity allowed is 999
CAS RN: 19350-66-4 | 產品號碼: B6316
3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid

* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | B6316 |
| 純度/分析方法 | >95.0%(T)(HPLC) |
| 分子式 / 分子量 | C__1__4H__1__9NO__6 = 297.31 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Air Sensitive |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 19350-66-4 |
| Reaxys-RN | 489397 |
| PubChem Substance ID | 468591034 |
| MDL編號 | MFCD00417306 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| 熔點 | 220 °C |
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS編碼* | 2933.39-000 |
-
Used Chemicals
-
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid [B6316]
- 2,3,5-Tri-O-benzyl-α/β-D-ribofuranose (1)
- 4-Dimethylaminopyridine (= DMAP) [D1450]
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (= EDCI) [D1601]
- Dichloromethane
- Sodium Carbonate [S0560]
- 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (= 4CzIPN)
- Methyl 4-Bromobenzoate [B0556]
- 2,2'-Bipyridyl (= bpy) [B0468]
- Nickel(II) Bromide Ethylene Glycol Dimethyl Ether Complex (= NiBr2・DME) [N1050]
- 1,4-Dioxane
-
Procedure
-
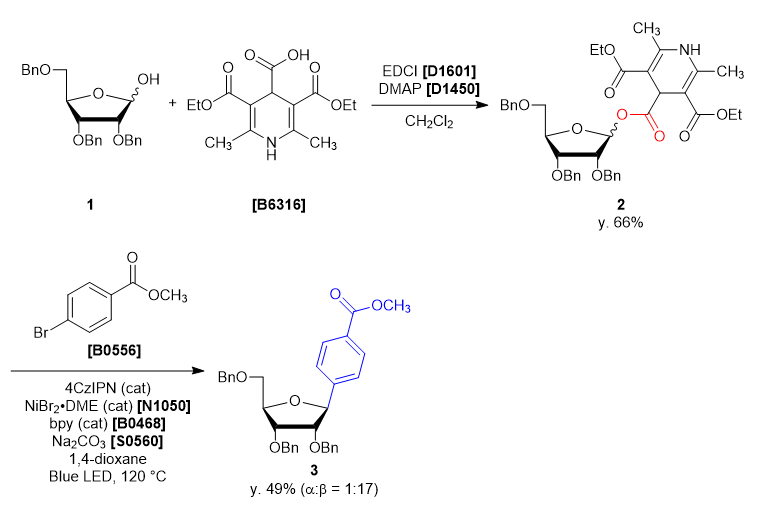
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%). -
-
Experimenter’s Comments
-
- NiBr2・DME was weighed in a nitrogen-filled glove box and dissolved in 1,4-dioxane completely using a sonication.
- 1,4-Dioxane was degassed with nitrogen for 30 min before use.
- Irradiation of visible light was performed with Kessil A160WE Tuna Blue 40W x 2.
- The reaction mixture was monitored by 1H NMR and UPLC.
- The α/β selectivity of 3 was 1:17.
-
Analytical Data
-
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
-
Lead Reference
-
- Diastereoselective Synthesis of Aryl C-Glycosides from Glycosyl Esters via C-O Bond Homolysis
-
Other Reference
-
- Highly stereoselective synthesis of aryl/heteroaryl-C-nucleosides via the merger of photoredox and nickel catalysis
文章/手冊
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜
很抱歉,您搜索的分析圖譜無法提供。




