Maximum quantity allowed is 999
CAS RN: 32005-36-0 | 產品號碼: B1374
Bis(dibenzylideneacetone)palladium(0)

| 包裝 | 價格 | 同一天 | 2-3个工作日 |
|---|---|---|---|
| 1G |
NT$3,520
|
20 | ≥60 |
| 5G |
NT$11,280
|
7 | ≥100 |
* 以上價格已含運費關稅等但一些需要海運以及乾冰運輸的產品除外,詳情請與
當地經銷商
洽詢。
* TCI會時常優化儲存條件,儲存溫度請以在線目錄為準,敬請留意。
| 產品號碼 | B1374 |
| 分子式 / 分子量 | C__3__4H__2__8O__2Pd = 575.02 |
| 外觀與形狀(20°C) | Solid |
| 儲存條件 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 儲存在惰性氣體下 | Store under inert gas |
| 應避免的情況 | Air Sensitive |
| 包裝和容器 | 1G-Glass Bottle with Plastic Insert (閲覽圖片) |
| CAS RN | 32005-36-0 |
| PubChem Substance ID | 87564299 |
| SDBS (AIST Spectral DB) | 50493 |
| MDL編號 | MFCD00051942 |
| Appearance | Brown to Black powder to crystal |
| Content (Palladium) | 16.5 to 20.5 % |
| 熔點 | 150 °C |
| 圖形表示 |

|
| 信號詞 | Warning |
| 危險性說明 | H302 + H312 + H332 : Harmful if swallowed, in contact with skin or if inhaled. H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防範說明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P261 : Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray. P270 : Do not eat, drink or smoke when using this product. P271 : Use only outdoors or in a well-ventilated area. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. P302 + P352 + P312 : IF ON SKIN: Wash with plenty of water.Call a POISON CENTER/doctor if you feel unwell. P304 + P340 + P312 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER/doctor if you feel unwell. |
| HS編碼* | 2843.90-000 |
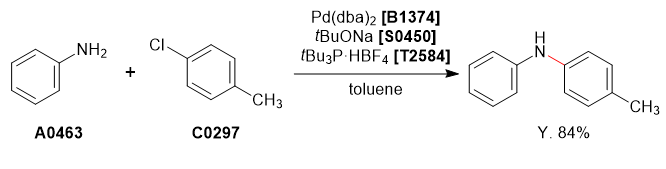
Used Chemicals
Procedure
Aniline (1.0 g, 10.7 mmol) and 4-chlorotoluene (1.4 g, 11.8 mmol) were dissolved in degassed toluene (10 mL) under nitrogen atmosphere. To this solution was added sodium tert-butoxide (1.54 g, 16.1 mmol), tri-tert-butylphosphonium tetrafluoroborate (31.1 mg, 0.1 mmol) and Pd(dba)2 (61.7 mg, 0.1 mmol, 1.5mol%). The reaction mixture was refluxed for 24 h and cooled to room temperature and quenched with H2O (10 mL). The organic phase was separated and washed with brine (10 mL), dried over MgSO4 (10 g), concentrated under reduced pressure to afford the crude product as a brown liquid. The crude product was purified by column chromatography on silica-gel (hexane : ethyl acetate = 95 : 5) to afford the desired product as a pale yellow crystalline solid (1.21 g, 6.6 mmol, 62%).
Experimenter’s Comments
Toluene was degassed by bubbling with N2 gas for 30 min.
The reaction mixture was analyzed by TLC (hexane : ethyl acetate = 1 : 1, Rf = 0.30) and GC.
Analytical Data (4-Methyl-N-phenylaniline)
1H NMR (400 MHz, CDCl3); δ 7.24-7.20 (t, J = 8.7 Hz, 3H), 7.07 (d, J = 8.7 Hz, 2H), 7.00-6.88 (m, 4H), 6.88-6.84 (m, 1H), 5.58 (s, 1H), 2.29 (s, 3H).
Lead Reference
- Tetramethoxybenzene is a Good Building Block for Molecular Wires: Insights from Photoinduced Electron Transfer
Used Chemicals
- 2-Chloro-m-xylene [C2164]
- 2-Methylphenylboronic Acid [M1313]
- Bis(dibenzylideneacetone)palladium (0) (Pd(dba)2) [B1374]
- 2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) [D5036]
- Tripotassium Phosphate (K3PO4)
- Toluene
- Ion-exchange water
Procedure
To a 3-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (66 mg, 0.115 mmol, 1.5 mol%), SPhos (94 mg, 0.229 mmol, 3.0 mol%), 2-methylphenylboronic acid (1.56 g, 11.5 mmol, 1.5 equiv.), tripotassium phosphate (4.87 g, 22.9 mmol, 3.0 equiv.) degassed toluene (15 mL), and ion-exchange water (1.5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 2-Chloro-m-xylene (1.0 mL, 7.64 mmol, 1.0 equiv.) was added one portion. The resulting mixture was stirred at reflux for 7 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane only) to afford the corresponding compound as a colorless oil (1.46 g, 97%).
Experimenter's Comments
i) Toluene was degassed by bubbling with nitrogen gas for 30 min.
ii) The reaction mixture was monitored by GC.Analytical Data(2,2',6-Trimethyl-1,1'-biphenyl)
1H NMR (400 MHz, CDCl3); δ 7.23-7.33 (m, 3H), 7.22-7.10 (m, 3H), 7.00-7.06 (m, 1H), 1.99 (s, 3H), 1.97 (s, 6H).
13C NMR (101 MHz, CDCl3); δ 141.0, 140.5, 135.8, 135.5, 129.9, 128.8, 127.2 127.0, 126.9, 126.0, 20.3, 19.4.
Lead Reference
- Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure
Used Chemicals
Procedure
-
To a 2-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (36 mg, 0.0633 mmol, 1.5 mol%), XPhos (60 mg, 0.127 mmol, 3.0 mol%), sodium tert-butoxide (811 mg, 8.44 mmol, 2.0 equiv.) and toluene (5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 4-chlorotoluene (0.5 mL, 4.22 mmol, 1.0 equiv.), and morpholine (0.55 mL, 6.33 mmol, 1.5 equiv.) were added in one portion. The resulting mixture was stirred at reflux for 6 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane : ethyl acetate = 9 : 1) to afford the corresponding compound as an orange solid (700 mg, 94%).
Experimenter's Comments
Toluene was degassed by bubbling with nitrogen gas for 30 min.
The reaction mixture was monitored by GC.Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.10 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 3.87 (t, J = 4.8 Hz, 4H), 3.11 (t, J = 4.8 Hz, 4H), 2.28 (s, 3H).
13C NMR (101 MHz, CDCl3); δ 149.1, 129.7, 129.5, 116.0, 66.9, 49.9, 20.4.
Lead Reference
- Expanding Pd-Catalyzed C−N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions
- Pd-Catalyzed Kumada-Corriu Cross-Coupling Reactions at Low Temperatures Allow the Use of Knochel-type Grignard Reagents


References
文章/手冊
SDS
請求的SDS不可用。
如需更多幫助,請聯繫我們 。
產品規格
檢驗報告(CoA)及其他文檔
示例 CoA
目前沒有該產品的 CoA 示例。
分析圖譜
很抱歉,您搜索的分析圖譜無法提供。





![(4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate (4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate](/medias/D5817.jpg?context=bWFzdGVyfHJvb3R8MjU2NzV8aW1hZ2UvanBlZ3xhRGxtTDJnMU5TODVNVEUyT0RJMk1qTTVNREEyTDBRMU9ERTNMbXB3Wnd8YmJkMWY4YTBjMWZkYTI1ZDRhZTVkNzRmZTk3YzM3N2FiZTYzNDUwNGJlNzEzMjJlODIzZDUyMzA0NWNiNThjMQ)

