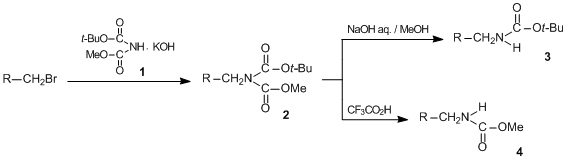
Maximum quantity allowed is 999
请选择数量
Fukuyama and co-workers have demonstrated various reactions using the N-substituted sulfonamide 5.3) Smooth reactions of 5 occur with alkyl halides under basic conditions and alcohols under Mitsunobu conditions to provide o-nitrobenzenesulfonyl (o-Ns) amines 6. The various o-Ns amines (Alloc, Boc, Cbz) 6 obtained from these reactions can be selectively deprotected, under the appropriate conditions, to afford the monoprotected amines 7 and 8. Furthermore, 7 can be converted to the primary amine in high yields via a second deprotection. 8 can be converted to the secondary amine in high yields by repeating the alkylation and deprotection process.4)

On the other hand, acetoxime O-(2,4,6-trimethylphenyl)sulfonate 9 has been reported as a useful electrophilic aminating agent. 9 reacts with Grignard reagents in the presence of a catalytic amount of magnesium chloride to afford primary amines in good yields.5)
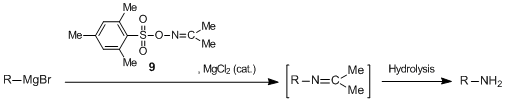
References
- 1)a) S. Gabriel, Ber. 1887, 20, 2224.

- 2)a) J. D. Elliott, J. H. Jones, J. Chem. Soc., Chem. Commun. 1977, 758.

- 3)T. Fukuyama, M. Cheung, T. Kan, Synlett 1999, 1301.

- 4)T. Kan, A. Fujiwara, H. Kobayashi, T. Fukuyama, Tetrahedron 2002, 58, 6267.

- 5)E. Erdik, M. Ay, Synth. React. Inorg. Met.-Org. Chem. 1989, 19, 663.

| 產品號碼 | B1648 |
| CAS RN | 18303-04-3 |
| 純度/分析方法 | >98.0%(T)(HPLC) |
| 產品號碼 | B2303 |
| CAS RN | 198572-71-3 |
| 純度/分析方法 | >98.0%(T)(HPLC) |
| 產品號碼 | C1757 |
| CAS RN | 245365-64-4 |
| 純度/分析方法 | >98.0%(T)(HPLC) |
| 產品號碼 | P2856 |
| CAS RN | 1293990-73-4 |
| 純度/分析方法 | >98.0%(T) |
添加到您的購物車
Product Availability by Store Location
Hours